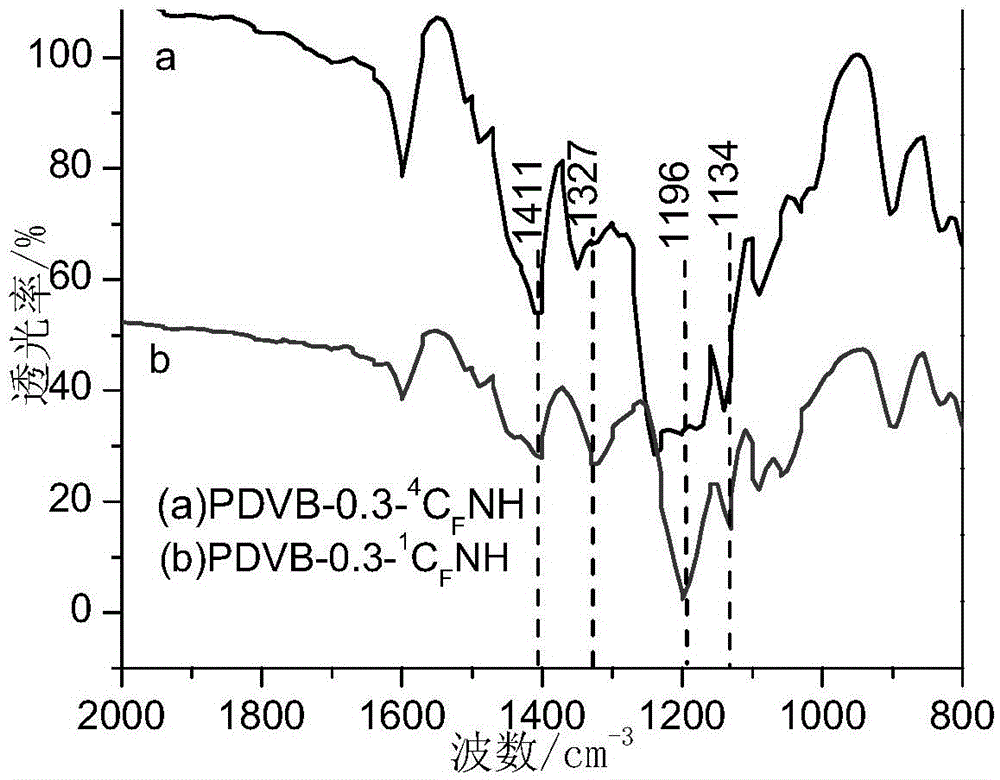
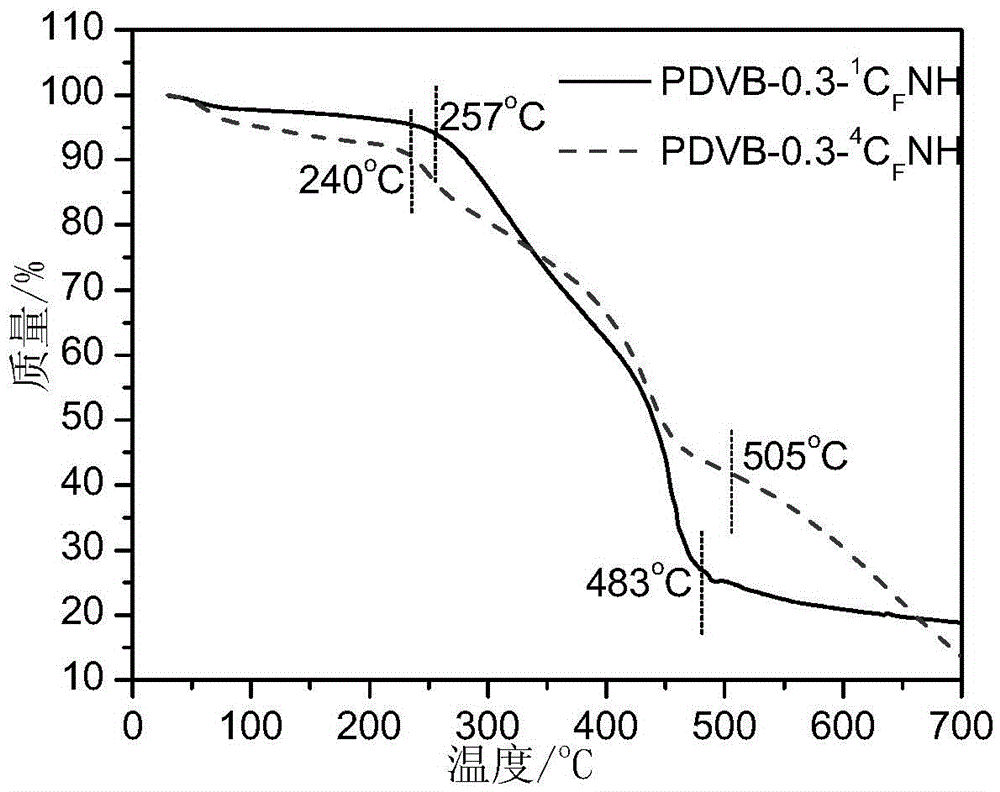
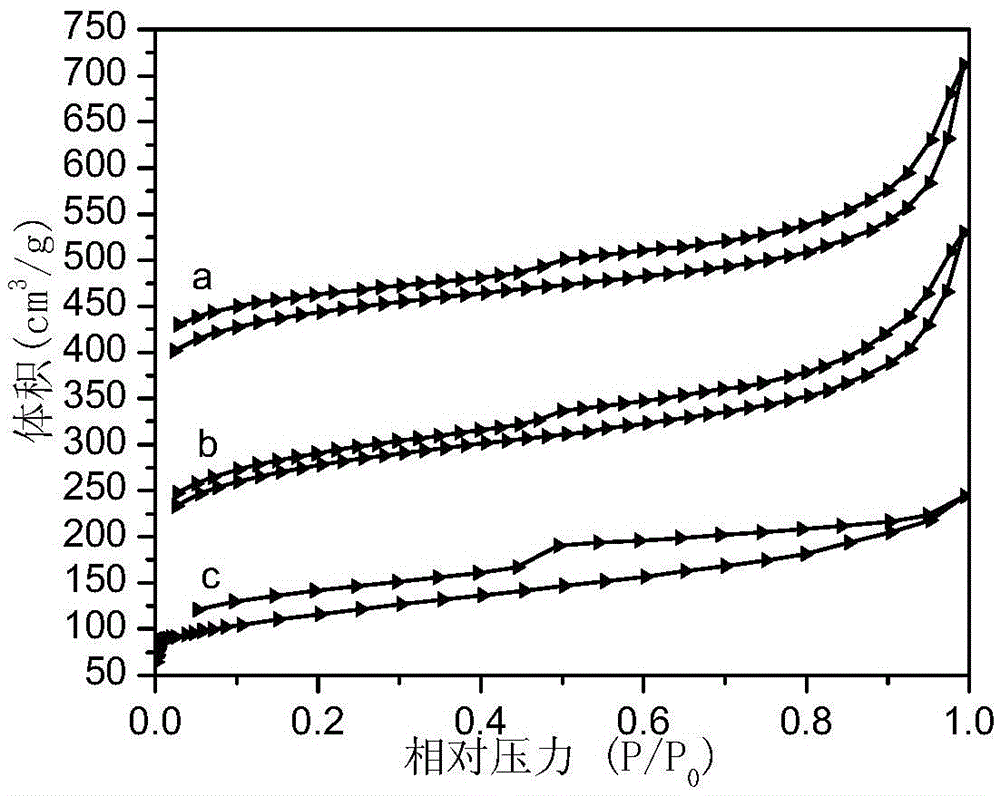
A kind of porous perfluoroalkyl sulfonylimide acidic resin and its application
A perfluoroalkyl sulfonylimide acidic and resin technology, which is applied in the field of coumarin synthesis, can solve the problem that the influence of the H site of the resin cannot be ruled out
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of porous trifluoromethanesulfonimide acidic resin comprises the following steps:
[0044] Synthesis of step 1 p-styrenesulfonyl trifluoromethylsulfonylimide sodium
[0045] The synthesis reaction scheme is as follows:
[0046]
[0047] In a 250mL double-necked round bottom flask, add 16.585g (0.08mol) of p-styrenesulfonyl chloride with a polymerization inhibitor, stir, add 120mL of refined 1,2-dichloroethane, add 13.230g (0.088mol) of three Fluoromethylsulfonamide, heated up to about 40°C to completely dissolve trifluoromethylsulfonamide, and another 19.021g was refined (C 2 h 5 ) 3 Add N (0.188mol) dropwise to a constant pressure funnel, raise the temperature to 60-70°C for 48 hours, cool, add about 40mL0.5mol / LH 2 SO 4 Acidification to remove excess (C 2 h 5 ) 3 N, separation, chloroform extraction, combined organic layers, first with Na 2 CO 3 Wash to alkaline, then wash to neutral with distilled water, concentrate, anhydro...
Embodiment 2
[0054] Example 2: Synthesis of Porous Perfluoroalkylsulfonylimide Acidic Resin Catalyzed 7-Hydroxy-4-methylcoumarin
[0055] The synthetic reaction of 7-hydroxyl-4 methylcoumarin is as follows:
[0056]
[0057] Add 0.55g (5mmol) resorcinol (pale yellow solid), 1.16g (10mmol) methyl acetoacetate (colorless liquid), and 0.62g PDVB-0.3- 4 C F NH (the molar weight of the loaded acid is 10% of the molar weight of resorcinol), the temperature was raised to 140°C for reaction, and the progress of the experiment was monitored by TLC for 2 hours. After the reaction was completed, it was in the form of a bright yellow powder solid, cooled to room temperature, poured into 20 mL of ice water and stirred, a large amount of solid was precipitated, filtered under reduced pressure, and a light yellow solid remained. Soxhlet extracted the solid with acetone at 78°C for 12 hours, and desolvated the extract to obtain a pale yellow solid, which was recrystallized from methanol to obtain a n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com