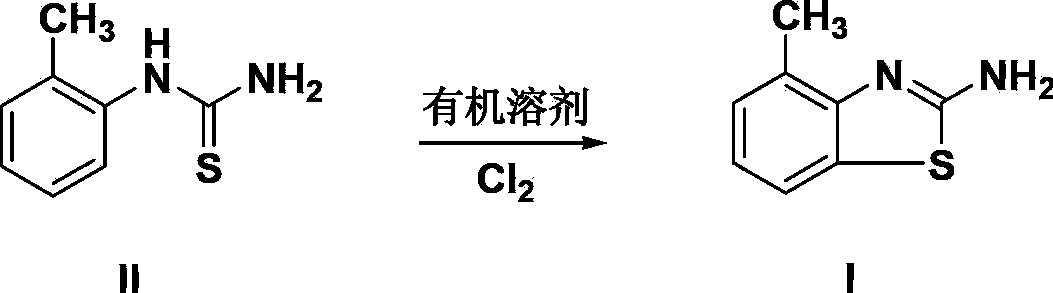
Preparation method of 4-methyl-2-amino benzothiazole
An aminobenzene and methyl technology, which is applied in the field of preparing 4-methyl-2-aminobenzothiazole, can solve the problem that it is difficult to obtain high-quality 4-methyl-2-aminobenzothiazole, and it is difficult to prepare high-quality 4-methyl-2-aminobenzothiazole. Tricyclazole requirements, failure to meet green and clean production and other problems, to achieve the effect of high product yield, good quality, and reduced raw material consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 35.0g (content 95%, 0.20mol) of o-toluenethiourea and 150mL of chloroform to a 500mL four-necked flask in turn, stir and cool down to about 10°C and start to pass chlorine gas at a speed of 0.06-0.02mol / h. Chlorine gas, keep the temperature at 10-20°C throughout the reaction process, stop the chlorine gas flow until the remaining ~3% of the reaction raw materials are traced by HPLC (no polychlorinated impurities are detected), and the reaction ends. Directly add 120ml of water in the flask, and recover the solvent by atmospheric distillation. Cool the remaining reaction liquid to 40-60°C, neutralize it with 30% sodium hydroxide aqueous solution to neutrality, cool to 10°C and filter, beat the filter cake twice with about 120ml water at 50-60°C, and filter at 30-35°C , and dried to obtain 32.2 g of 4-methyl-2-aminobenzothiazole, content 98.6%, yield 96.8%, mp 136-138°C.
Embodiment 2
[0025] The solvent used for the reaction is changed into carbon tetrachloride, and other items such as charging amount, reaction conditions and post-treatment operation are the same as in Example 1. 32.14 g of 4-methyl-2-aminobenzothiazole was obtained, the content was 98.7%, the yield was 96.7%, and the mp was 136-138°C.
Embodiment 3
[0027] Change the chlorine flow rate to 0.12-0.08mol / h, and stop the chlorine gas flow when the remaining ~4.5% of the reaction raw materials are tracked by HPLC (no polychlorinated impurities are detected), and other items and post-treatment operations are the same as in Example 1. 31.8 g of 4-methyl-2-aminobenzothiazole was obtained, the content was 98.1%, the yield was 95.0%, and the mp was 136-138°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com