Method of detecting content of 1,1-dichloroacetone in drinking water by GC/MS combination
A technology of dichloroacetone and drinking water, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as not having qualitative analysis functions, and achieve the effect of small relative standard deviation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: terminator selection
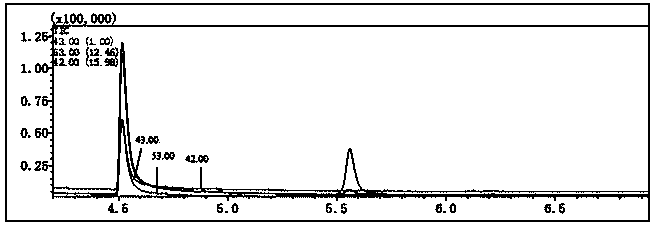
[0033] Usually the residual chlorine in drinking water after disinfection is between 0.05 ~ 4.0mg / L, and there will be a relatively high content of residual chlorine in the test water samples generated by DBPs, and it is necessary to add a reducing terminator to eliminate the strong Oxidative residual chlorine terminates the chlorination reaction process. In order to avoid the influence of the terminator on the stability of DCAce as much as possible. The effect of commonly used terminators on the stability of DCAce needs to be investigated.
[0034] Prepare multiple water samples of DCAce with a concentration of 100 μg / L (preparation method: take an appropriate amount of 5 mg / L DCAce standard, place it in a brown volumetric flask, and prepare a standard solution with a mass concentration of 10 μg / mL with deionized water, Then dilute the standard solution with ultrapure water to the required concentration), select one of the water s...
Embodiment 2
[0035] Embodiment 2: the selection of extractant
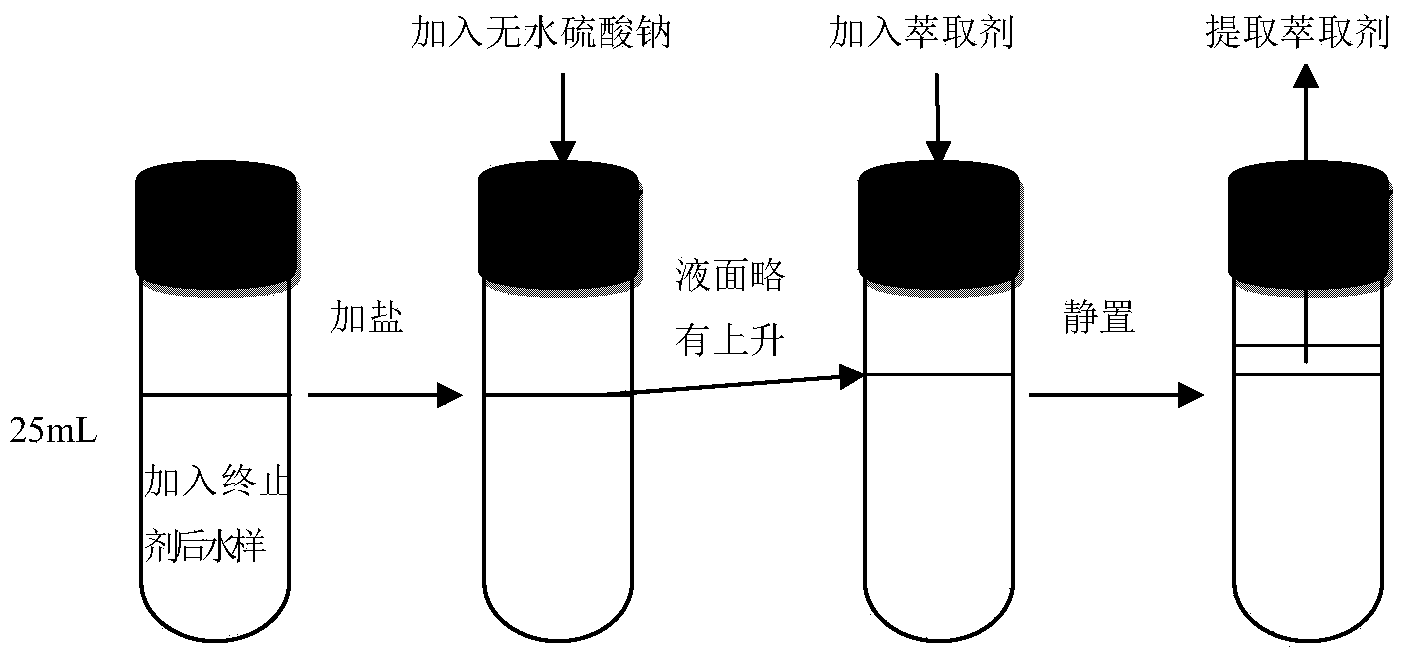
[0036]First, pass 100 μg / L of DCAce water sample (preparation method is the same as in Example 1) through a 0.22 μm microporous membrane, then add 8 g of anhydrous sodium sulfate to a glass bottle containing 25 mL of water sample, and place it on a shaker to At a speed of 720r / min, oscillate until the salt is completely dissolved, the solution is transparent, and the liquid level rises slightly; after that, add 2mL of extractant, place it on the shaker at a speed of 720r / min, shake for 5min, remove the sample bottle, and Set aside for 5min, take the upper layer extract, and carry out the determination of GC / MS. In this experiment, two kinds of extractants (methyl tert-butyl ether and ethyl acetate) were used, and the extraction effects of the two were compared, that is, the one with higher recovery rate was superior. The standard addition recovery rate of methyl tert-butyl ether is above 90%, and the standard addition recover...
Embodiment 3
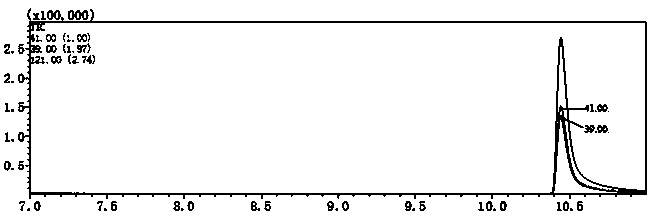
[0037] Embodiment 3: the selection of internal standard substance
[0038] In the present invention, 1,2-dibromopropane and n-decane were used as internal standard substances for comparative study, which were dissolved in methyl tert-butyl ether and prepared step by step to a concentration of 150 μg / L. Used as extractant in follow-up experiments. Configure multiple 100μg / L DCAce water samples (the preparation method is the same as in Example 1), take 25mL water samples and add them to 40mL glass bottles, then add 8g of anhydrous sodium sulfate, place on the shaker at 720r / min Shake until the salt is completely dissolved, the solution is transparent, and the liquid level rises slightly; after that, add 2 mL of extractant A (containing 150 μg / L 1,2-dibromopropane) and extractant B (containing 150 μg / L decane), placed on an oscillator at a speed of 720r / min, oscillated for 5 minutes, removed the sample bottle, and stood still for 5 minutes, then took the upper layer extractant f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com