Application of miltirone in preparation of antitumor drugs
An anti-tumor drug, the technology of tanshinone, is applied in the directions of anti-tumor drugs, drug combinations, separation/purification of carbonyl compounds, etc., to achieve the effects of inhibiting tumor cell proliferation, sensitizing the formation of new blood vessels, and preventing tumors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: In order to further understand the present invention, the preparation of tanshinone with the structure of formula I will be described in detail below. Here, the present invention provides a preparation method or synthesis technology.
[0040] Salvia miltiorrhiza medicinal material (3kg) is 95% ethanol aqueous solution extraction with the ethanol mass percentage of 8 times weight, each 24Kg salvia miltiorrhiza medicinal material, altogether 3 times, merges and obtains ethanol extract, and ethanol extract is with sherwood oil, dichloromethane, n-butanol Sequential extraction yielded a petroleum ether layer, a dichloromethane layer, and n-butanol layer, respectively.
[0041] The dichloromethane layer (70 g) was dissolved in 100 mL of ethyl acetate, and 70 g of 200-300 mesh chromatography silica gel was added to mix the sample to obtain the mixed sample. Wet-pack the column with 300g 200-300 mesh chromatography silica gel, add the mixed sample, and fix the colum...
Embodiment 2
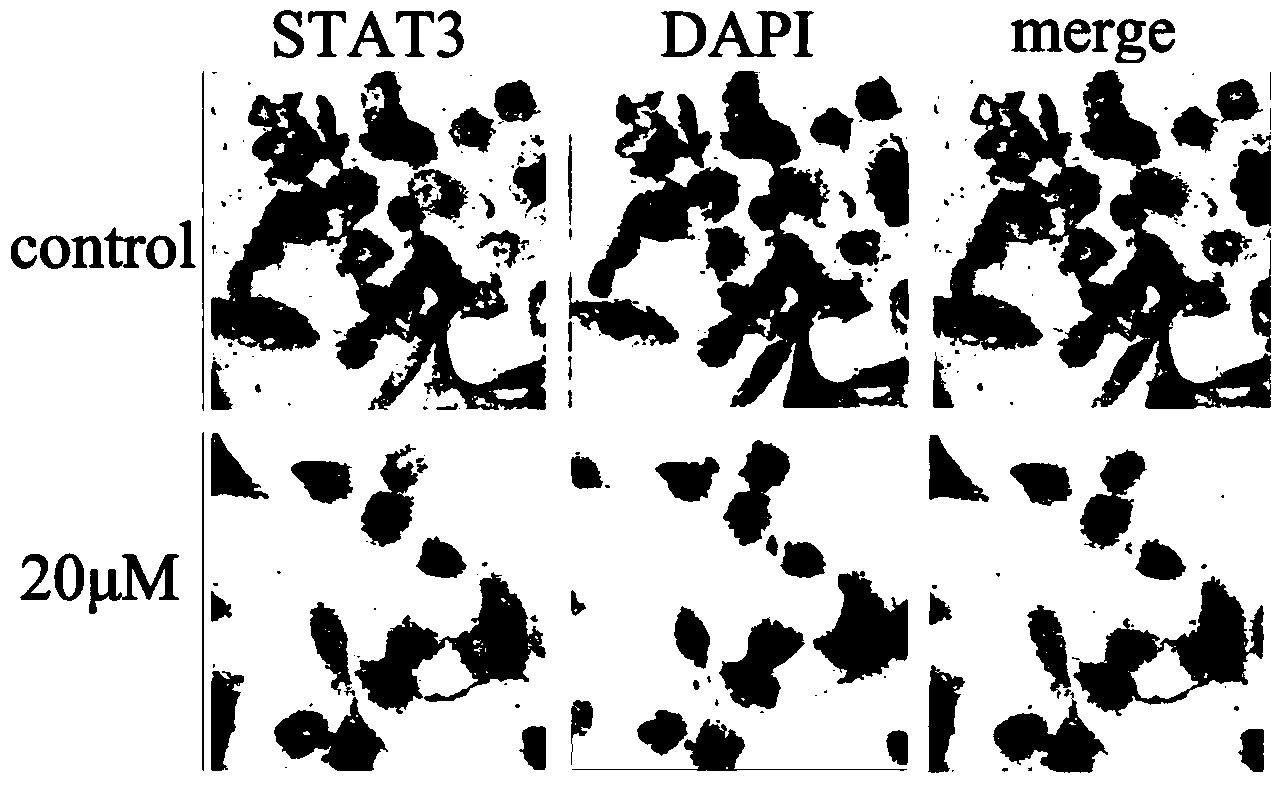
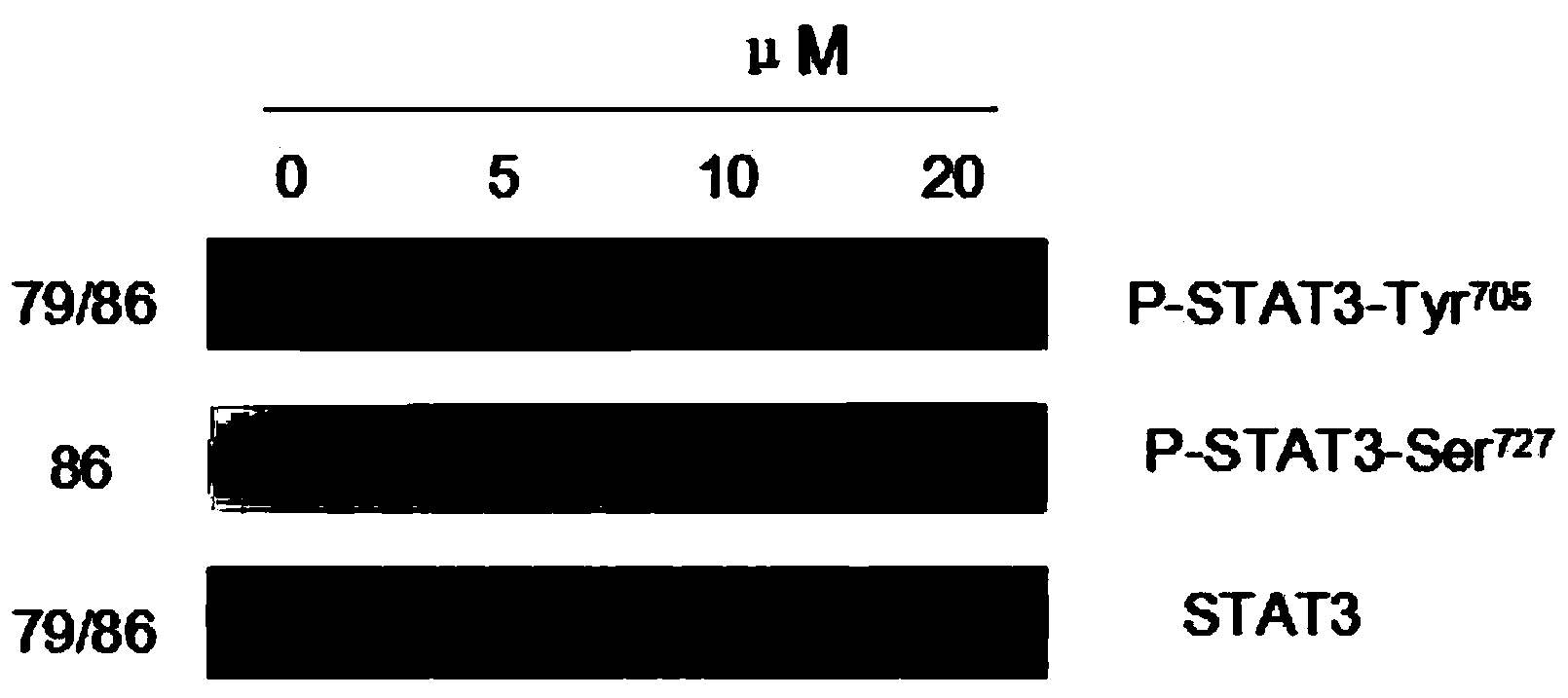
[0045] Example 2: Tanshinone inhibits lung cancer cell NCI-H1975p-stat3-tyr (705) Activation, no inhibition of p-stat3-ser
[0046] Western blot detection of p-STAT3-tyr in lung cancer cells NCI-H19754H (0, 5 μM, 10 μM, 20 μM) treated (705) , p-STAT3-ser (727) , STAT3[(antibodies were purchased from CST Company (CellSignalingTechnology, Inc)], the experimental results showed that with the increase of concentration, p-STAT3-tyr (705) showed an obvious downward trend, while p-STAT3-ser (727) There is no change, see the specific results figure 1 .
[0047] The specific implementation method is as follows:
[0048] Take the lung cancer cells NCI-H1975 in the logarithmic growth phase, prepare a single cell suspension, and count 1×10 6 Add each bottle into a 5mL culture flask. After the cells adhere to the wall the next day, add drugs of different concentrations (0, 5μM, 10μM, 20μM). After 4h, collect the cell samples into a 15ml centrifuge tube, 800rpm / 5min, discard the super...
experiment example 3
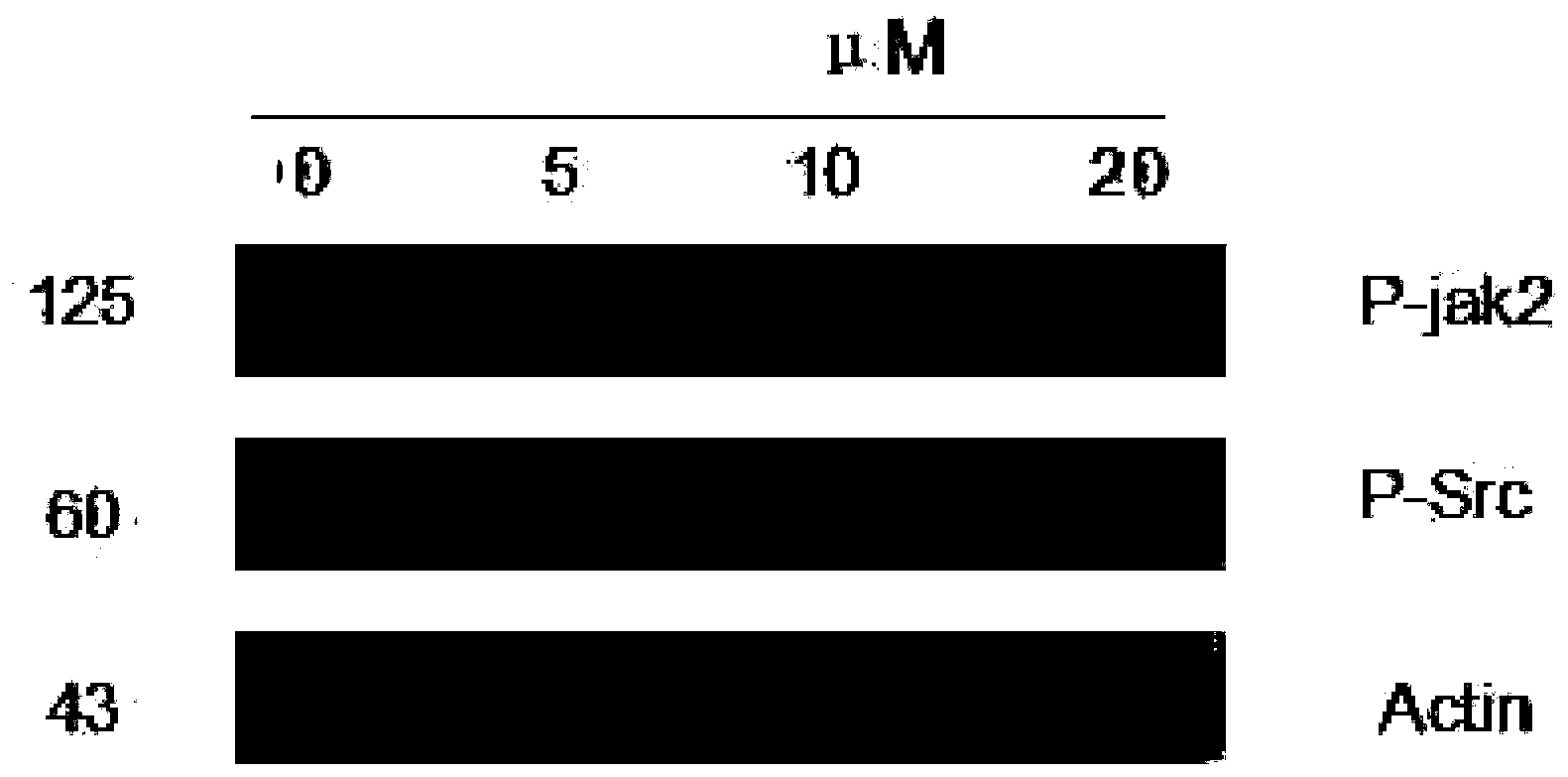
[0049] Experimental example 3: Effect of tanshinone on the phosphorylation levels of Stat3 upstream regulatory kinases P-Src and P-jak2 in lung cancer cell NCI-H1975.
[0050] After lung cancer cells NCI-H1975 (0, 5 μM, 10 μM, 20 μM) were treated for 4 hours, P-jak2, P-Src, and β-actin were detected by Western blot technique [(antibodies were purchased from CST Company (Cell Signaling Technology, Inc)], the experimental results It shows that the upstream kinases of STAT3 have no obvious changes, and the specific results are shown in figure 2 .
[0051] The specific implementation method is basically the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com