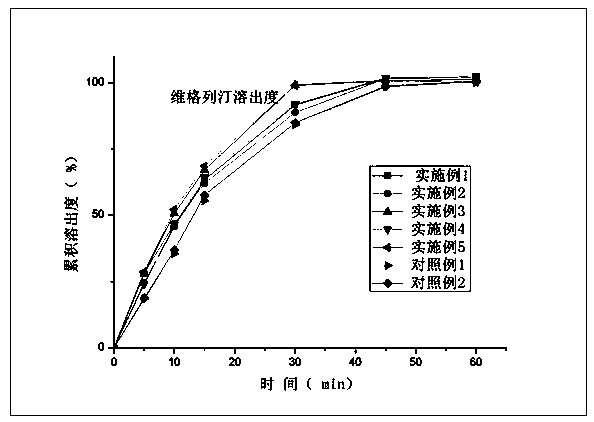
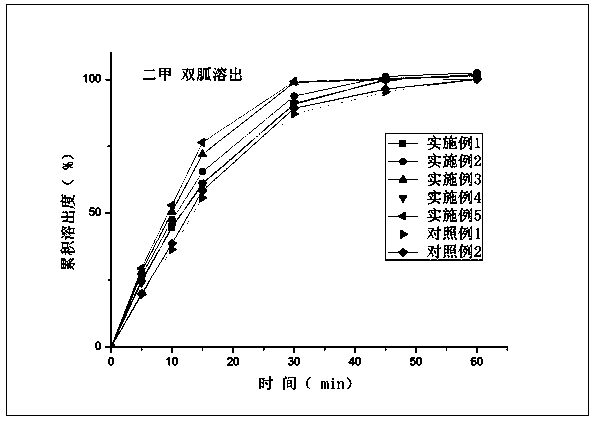
Compound composition of vildagliptin and melbine and preparation method thereof
A technology for metformin and a composition is applied to the compound tablet composition containing vildagliptin and metformin and the field of preparation thereof, and can solve the problems of difficult mixing of main drugs, poor compressibility of vildagliptin, large dose of metformin and the like , to achieve the effect of being suitable for clinical application, with small batch-to-batch variability and small content variability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
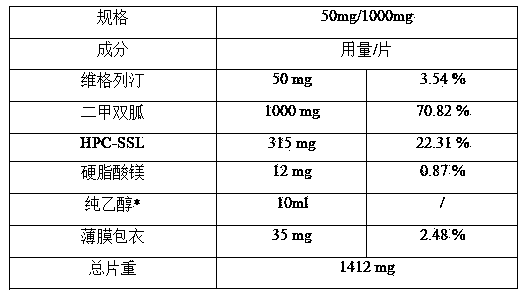
[0078] Example 1 wet granulation
[0079] Table 1 Example 1 prescription summary
[0080]
[0081] * Removed during processing.
[0082] Specific process: Grind metformin needle crystals and pass through a 100-mesh sieve for later use; pass hydroxypropyl cellulose through a 60-mesh sieve for later use. Pass metformin and hydroxypropyl cellulose through a 60-mesh sieve 10 times, mix evenly, add an appropriate amount of ethanol to prepare the soft material, and then use a 30-mesh sieve to granulate. The prepared granules were dried in an oven at 60°C. Pass the dried granules through a 36-mesh sieve for granulation. Mix the sized granules with the prescribed amount of vildagliptin and magnesium stearate for 15 minutes, and mix well. According to the prescription quantity, the mixed material was weighed, and a special-shaped punch of 20mm×10mm was used for tableting, keeping the hardness of the short diameter at 150N.
[0083]
Embodiment 2
[0084] Example 2 dry granulation
[0085] Table 2 Example 2 prescription summary
[0086]
[0087] * Removed during processing.
[0088] Specific process: Grind metformin needle crystals and pass through a 100-mesh sieve for later use; pass hydroxypropyl cellulose through a 60-mesh sieve for later use. The magnesium stearate of metformin, hydroxypropyl cellulose, and 50% prescription amount is passed through a 60-mesh sieve 10 times, and mixed uniformly. The mixed materials are punched into special-shaped tablets of 20 mm × 10 mm, and then the compressed tablets are crushed to a certain particle size with a mortar. Pass the pulverized particles through a 30-mesh sieve to ensure that the particle size of the dry granulation does not exceed 560 μm (30-mesh sieve). Mix the sized granules with the prescribed amount of vildagliptin and magnesium stearate for 15 minutes, and mix well. According to the prescription quantity, the mixed material was weighed, and a special-shap...
Embodiment 3
[0091] Table 3 Example 3 prescription summary
[0092]
[0093] * Removed during processing.
[0094] Specific process: Dissolve the prescribed amount of metformin and hydroxypropyl cellulose in 90% ethanol solution, and remove the solvent by rotating the solution at 60°C and 40 rpm. The resulting solid was scraped off and dried at 60°C. The dried material is crushed to a certain particle size with a mortar. Pass the pulverized particles through a 30-mesh sieve to ensure that the particle size does not exceed 560 μm (30-mesh sieve). Mix the sized granules with the prescribed amount of vildagliptin and magnesium stearate for 15 minutes, and mix well. According to the prescription quantity, the mixed material was weighed, and a special-shaped punch of 20mm×10mm was used for tableting, keeping the hardness of the short diameter at 150N.
[0095]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent viscosity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com