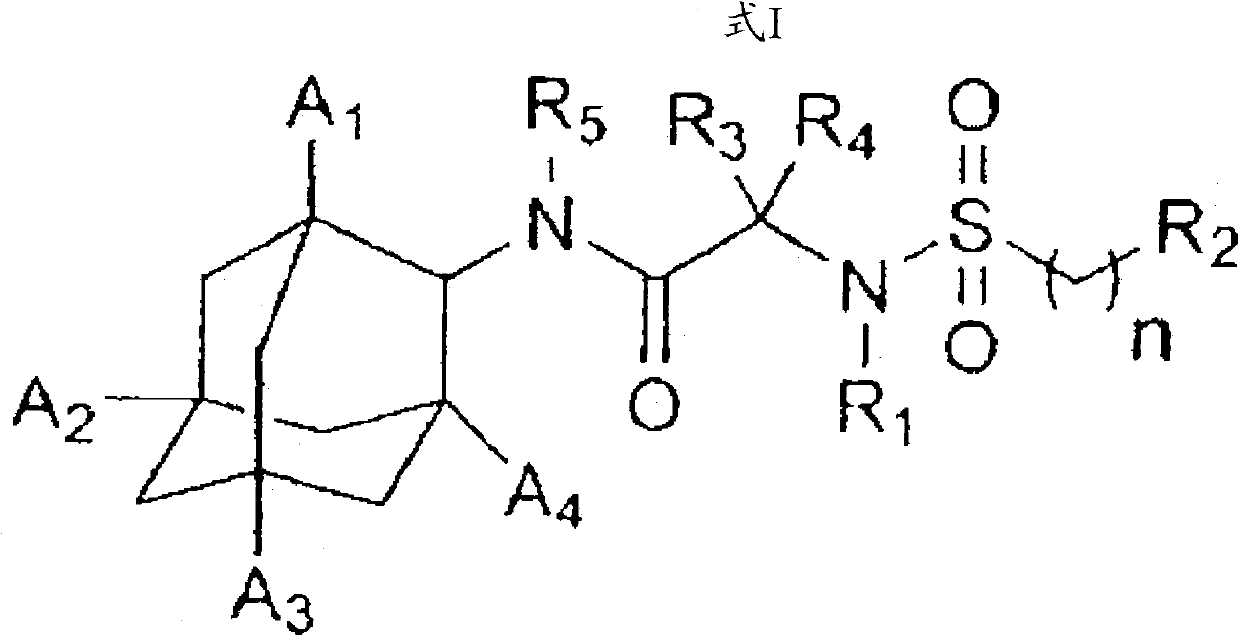
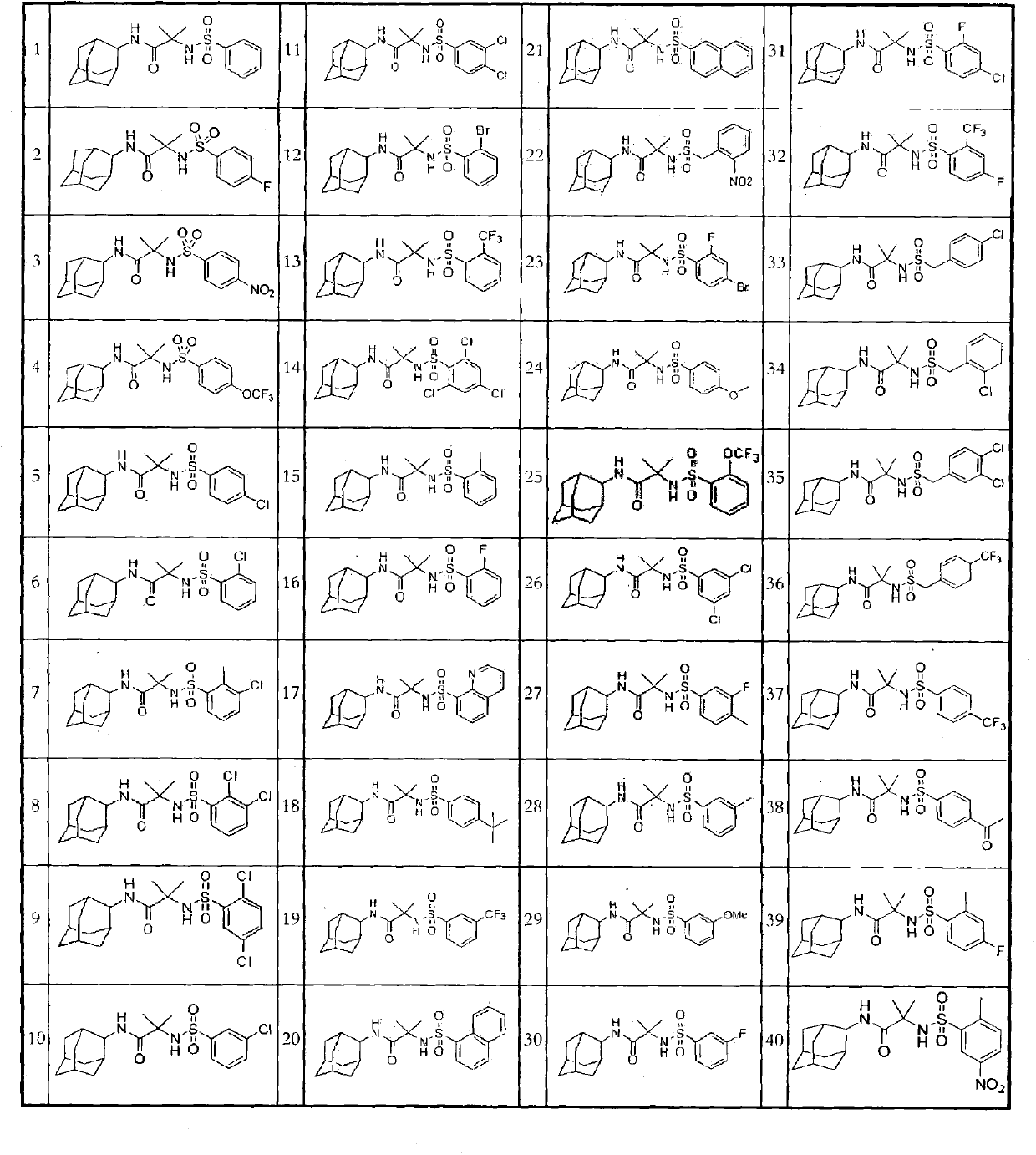
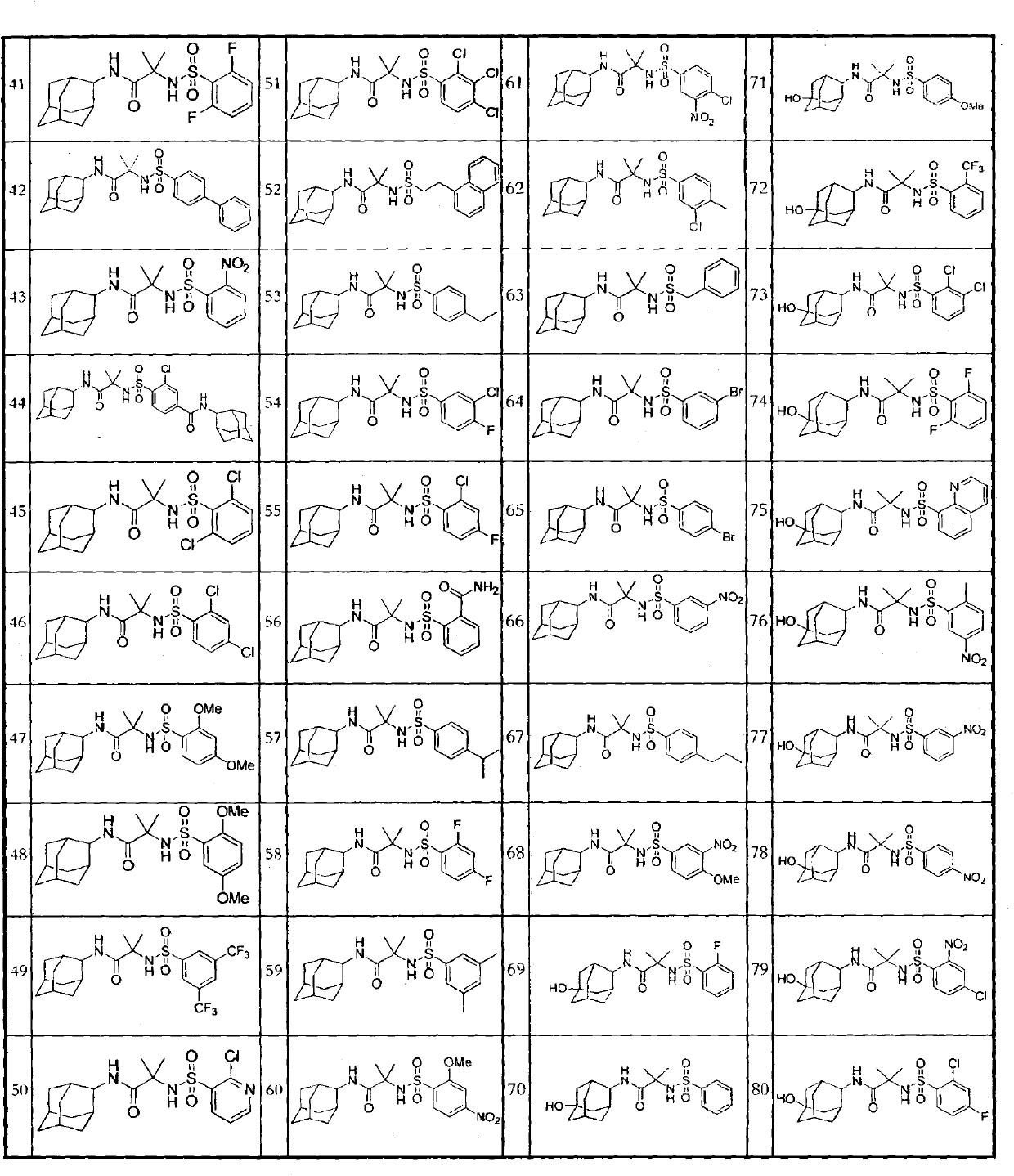
A compound for inhibiting 11ss-hydroxy steroid dehydrogenase 1, and a pharmaceutical composition comprising the same
A compound and solvate technology, applied in the field of compounds for inhibiting 11β-hydroxysteroid dehydrogenase 1 and pharmaceutical compositions containing the compounds, can solve the problems of increased 11βHSD1 activity and other problems, and achieve excellent activity and solubility, formulation and Transfer effective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0063] Preparation Example 1: Synthesis of N-(adamantan-2-yl)-2-methyl-2-(phenylsulfonamido)propionamide (compound 1)
[0064]
[0065] Put 2-amino-2-methylpropionic acid (3.0g, 29.1mmol) and methanol (45ml) into a 100-ml flask, set an ice bath, and slowly add thionyl chloride (4.25ml, 58.2mmol). After the addition is complete, remove the ice bath. The mixture was stirred for 3 hours at room temperature. The obtained product was vacuum distilled to remove the solvent, and then ether (30 ml) was added, stirred at room temperature for 30 minutes, and filtered. The solid obtained by filtration was dried in an oven at 60°C to obtain methyl 2-amino-2-methylpropionate hydrochloride (4.2 g, 94%).
[0066] 1 H NMR(400MHz, DMSO-d 6 )δ8.75(s, 3H), 3.75(s, 3H), 4.05(m, 1H), 1.45(s, 6H).
[0067]
[0068] Into a 50-mL flask, charge 2-amino-2-methylpropionic acid methyl ester hydrochloride (400 mg, 2.61 mmol) and dichloromethane (10 mL), and charge triethylamine (1.46 ml, 10.47 mmol) ), and ...
preparation Embodiment 2
[0076] Preparation Example 2: Synthesis of N-(adamantan-2-yl)-2-methyl-2-(4-fluorobenzenesulfonamido)propionamide (compound 2)
[0077] The target compound was obtained in the same manner as described in Preparation Example 1 of Example 1, except that 2-(4-fluorobenzenesulfonamido)-2-methylpropionic acid was used.
[0078]
[0079] 1 H NMR(400MHz, CDCl 3 )δ7.90-7.95(m, 2H), 7.18-7.23(m, 2H), 6.65(d, J=6.8Hz, -NH-CO-), 5.40(s, -NH-SO 2 ), 3.97 (d, J = 8.0 Hz, 1H), 1.67-1.90 (m, 14H), 1.46 (s, 6H).
preparation Embodiment 3
[0080] Preparation Example 3: Synthesis of N-(adamantan-2-yl)-2-methyl-2-(4-nitrobenzenesulfonamido)propionamide (compound 3)
[0081] The target compound was obtained in the same manner as described in Preparation Example 1 of Example 1 except that 2-(4-nitrobenzenesulfonamido)-2-methylpropionic acid was used.
[0082]
[0083] 1 HNMR(400MHz, CDCl 3 )δ8.37(dt, J=2.4, 8.8Hz, 2H), 8.10(dt, J=2.4, 9.2Hz, 2H), 6.36(d, J=7.6Hz, -NH-CO-), 5.85(s , -NH-SO 2 ), 3.96 (d, J=7.6Hz, 1H), 1.67-1.89 (m, 14H), 1.50 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com