Method for synthesizing asymmetric cyclotetrasiloxane
The technology of a cyclotetrasiloxane and a synthesis method, which is applied in the direction of silicon organic compounds and the like, can solve the problems of difficult separation and difficult to obtain high-purity asymmetric cyclotetrasiloxane, and achieves efficient synthesis, easy separation and purification, and reaction. mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 1, 1, 3, 3-tetramethyl-5, 7-diphenyl-5, 7-divinylcyclotetrasiloxane
[0028] 0°C, a solution of 0.1 g of 1, 1, 3, 3-tetramethyldisiloxane-1, 3-diol sodium salt in 10 ml of tetrahydrofuran was added dropwise to a solution containing 1.0 g of 1, 3-diiodo -1, 3-diphenyl-1, 3-divinyldisiloxane and 10 ml tetrahydrofuran in a 50 ml three-neck flask, stirred for 8 hours, then added 10 ml deionized water and 10 ml n-hexane for extraction , washed the organic layer with water until neutral, and dried over anhydrous sodium sulfate. Distilled under reduced pressure to obtain a colorless liquid, i.e. 1, 1, 3, 3-tetramethyl-5, 7-diphenyl-5, 7-divinylcyclotetrasiloxane, yield 65%, product purity 96%.
Embodiment 2
[0029] Example 2: Preparation of 1, 1, 3, 3-tetravinyl-5, 7-diphenylcyclotetrasiloxane
[0030] -20°C, add 200 g of 1, 1, 3, 3-tetravinyldisiloxane-1, 3-diol in 200 ml of ether solution dropwise to 280 g of 1, 3-dichloro- 1, 3-diphenyldisiloxane, 130 grams of diethylamine and 200 milliliters of diethyl ether in a 1000 milliliter three-neck flask, after stirring for 2 hours, add 400 milliliters of deionized water for extraction, wash the organic layer with water until neutral , dried over anhydrous sodium sulfate. Distillation under reduced pressure gave a colorless liquid, namely 1, 1, 3, 3-tetraethenyl-5, 7-diphenylcyclotetrasiloxane, with a yield of 90% and a product purity of 98%.
Embodiment 3
[0031] Example 3: Preparation of 1, 3, 5, 5, 7, 7-hexamethylcyclotetrasiloxane
[0032] 10°C, 50 g of 1, 1, 3, 3-tetramethyl-1, 3-disilanol in 200 ml of 1, 4-dioxane solution was added dropwise to 55 g of 1, 3- Dichloro-1,3-dimethyldisiloxane, 50 g of pyridine and 200 ml of 1,4-dioxane in a 1000 ml three-necked flask, stirred for 15 hours, then added 200 ml of deionized water for extraction , washed the organic layer with water until neutral, and dried over anhydrous sodium sulfate. Distillation under reduced pressure gave a colorless liquid, namely 1, 3, 5, 5, 7, 7-hexamethylcyclotetrasiloxane, with a yield of 70% and a product purity of 97%.
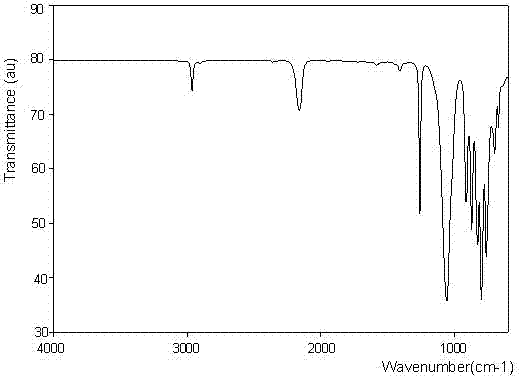
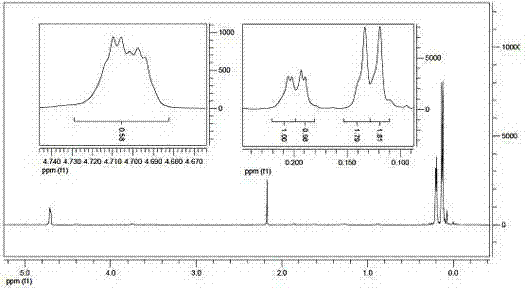
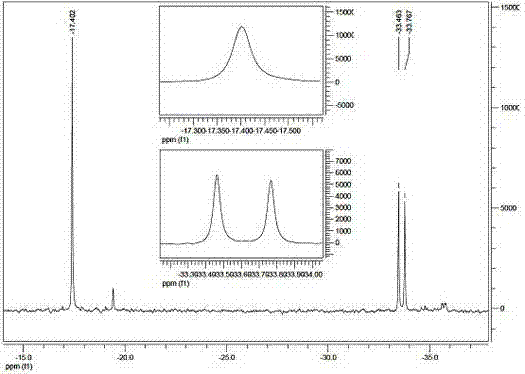
[0033] The infrared spectrum of 1, 3, 5, 5, 7, 7-hexamethylcyclotetrasiloxane is shown in figure 1 Shown, the gas chromatography-mass spectrum of 1, 3, 5, 5, 7, 7-hexamethylcyclotetrasiloxane is as figure 2 Shown, the 1, 3, 5, 5, 7, 7-Hexamethylcyclotetrasiloxane proton nuclear magnetic resonance spectrogram is as image 3 Shown, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com