Analysis method for determining oligomerization thelenota ananas glycosaminoglycan content
A technology of ginseng glycosamine and pineapple, applied in the analysis field of determining the content of oligomeric pineapple ginseng glycosaminoglycans
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] [embodiment 1] the selection of chromatographic column
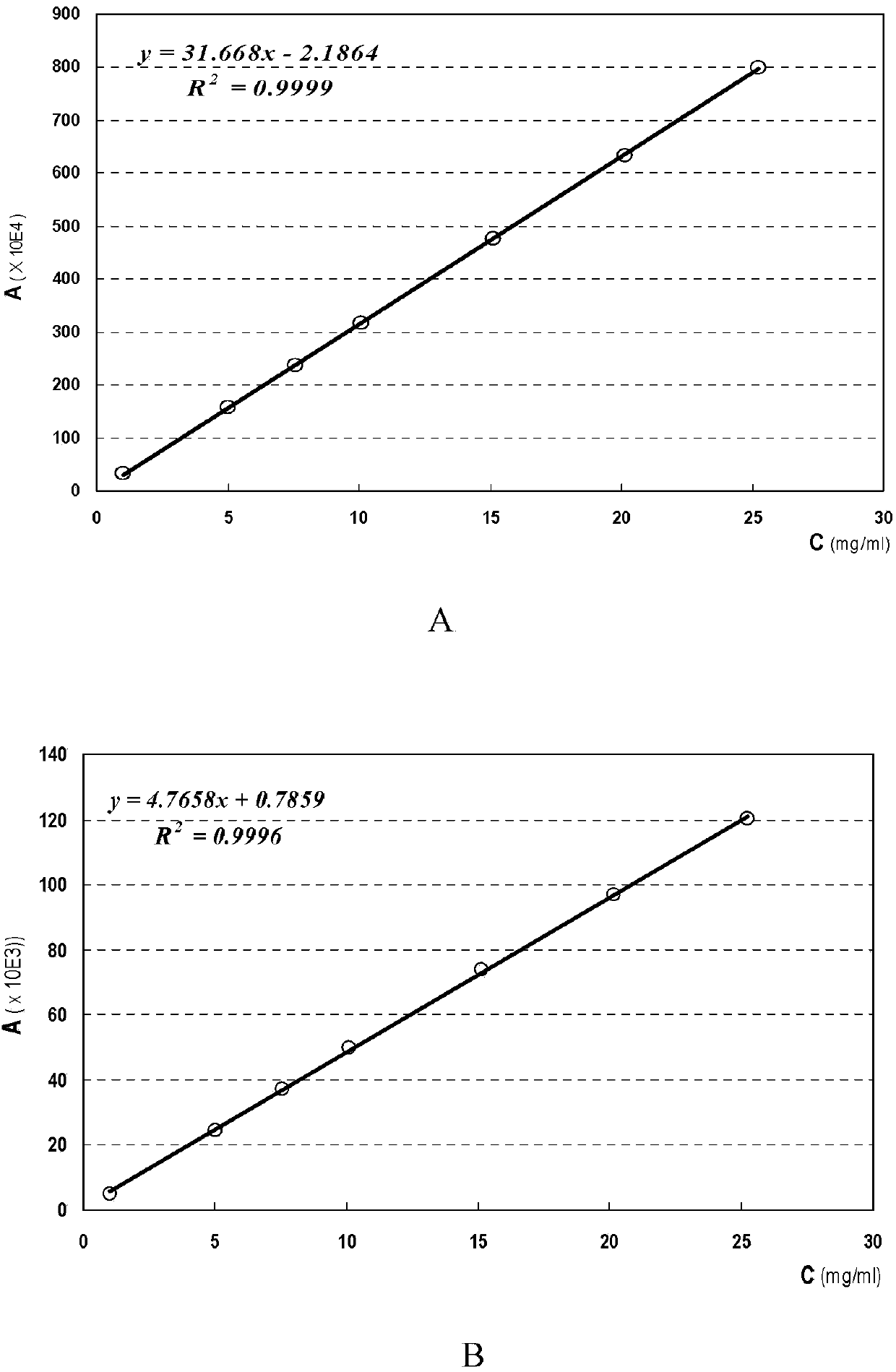
[0029] Chromatographic conditions and system suitability: Gel chromatographic columns, Shodex Ohpak SB-804HQ, Viscotek, TOSOHTSK-gel, Agilent PL Aquagel-OH and other four gel chromatographic columns were selected respectively. Use 0.1mol / L sodium chloride as the mobile phase; the flow rate is 0.5ml / min; the column temperature is 35°C; the temperature of the differential detector is 35°C. The number of theoretical plates should not be less than 1000 based on the calculation of the D2 peak of dextran.
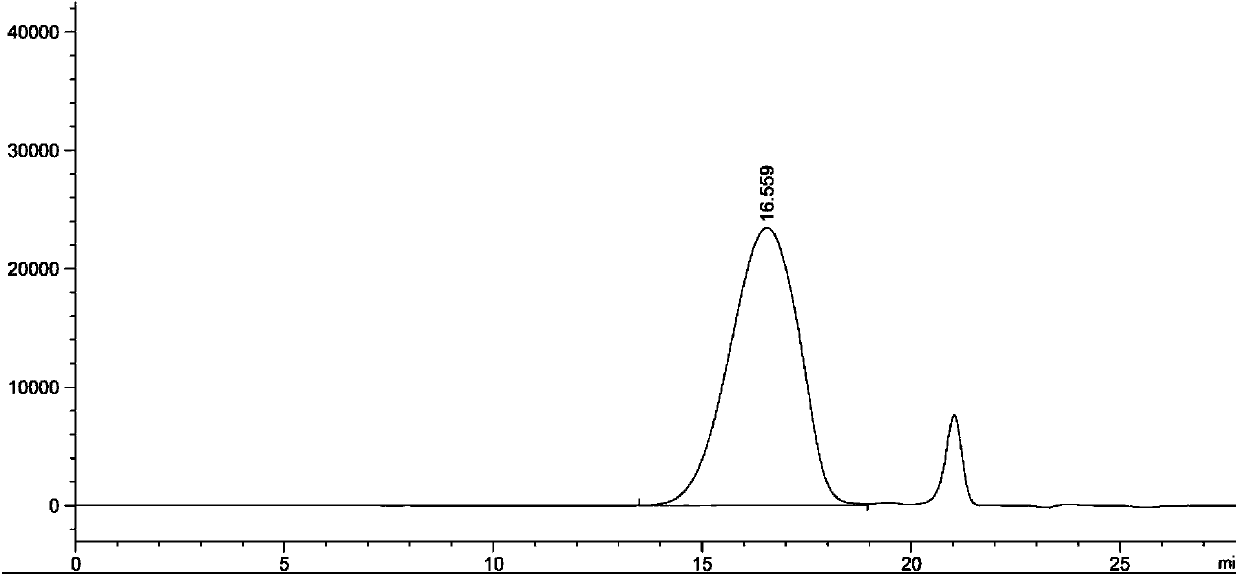
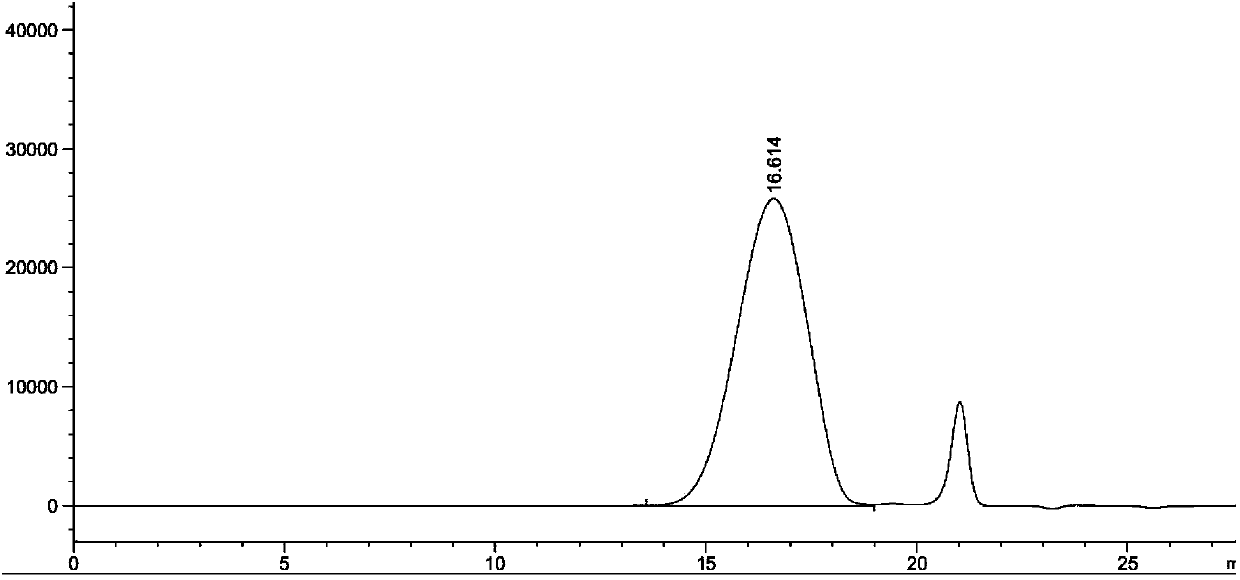
[0030] Determination method: Freeze-dried fengshensu preparation for injection, batch number: 20071201, take the contents, accurately weigh an appropriate amount (about 100 mg of fengshensu), put it into a 10ml measuring bottle, add 0.1mol / L sodium chloride solution Dissolve, dilute to the mark, shake well, filter, take the subsequent filtrate as the test solution; take another appropriate amount of fengshensu refer...
Embodiment 2
[0032] [embodiment 2] the selection of flow rate
[0033] Chromatographic conditions and system suitability: Shodex Ohpak SB-804HQ gel chromatography column; 0.10mol / L sodium chloride as the mobile phase; flow rates are 0.2ml / min, 0.3ml / min, 0.5ml / min, 0.7ml / min, 0.8ml / min, the column temperature is 35°C; the differential detector temperature is 35°C. The number of theoretical plates should not be less than 1000 based on the calculation of the D2 peak of dextran.
[0034] Determination method: Freeze-dried fengshensu preparation for injection, batch number: 20071201, take the contents, accurately weigh an appropriate amount (about 100 mg of fengshensu), put it into a 10ml measuring bottle, add 0.1mol / L sodium chloride solution Dissolve, dilute to the mark, shake well, filter, take the subsequent filtrate as the test solution; take another appropriate amount of fengshensu reference substance that has been dried under reduced pressure with phosphorus pentoxide to constant weig...
Embodiment 3
[0036] [Example 3] mobile phase ratio selection
[0037] Chromatographic conditions and system suitability: Shodex Ohpak SB-804HQ gel chromatography column; 0.07mol / L, 0.08mol / L, 0.10mol / L, 0.12mol / L, 0.13mol / L sodium chloride as the mobile phase; The flow rate is 0.5ml / min; the column temperature is 35°C; the differential detector temperature is 35°C. The number of theoretical plates should not be less than 1000 based on the calculation of the D2 peak of dextran.
[0038] Determination method: Freeze-dried fengshensu preparation for injection, batch number: 20071201, take the contents, accurately weigh an appropriate amount (about 100 mg of fengshensu), put it into a 10ml measuring bottle, add 0.1mol / L sodium chloride solution Dissolve, dilute to the mark, shake well, filter, take the subsequent filtrate as the test solution; take another appropriate amount of fengshensu reference substance that has been dried under reduced pressure with phosphorus pentoxide to constant weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com