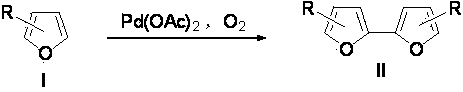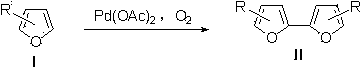Synthetic method for furan coupling compound
A synthetic method and furan coupling technology, which is applied in the field of synthesis of furan coupling compounds, achieves the effects of high regioselectivity, wide substrate adaptability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] IIa
[0016] In a 10mL single-necked flask was added Pd(OAc) 2 (22.4 mg, 10 mmol %), sealed and evacuated, replaced the air in the bottle with an oxygen balloon, and filled the flask with oxygen. Then, DMSO (1.5 mL) and TFA (75 μL, 1 mmol) were sequentially added to the flask via syringe under oxygen atmosphere, and the reaction mixture was stirred homogeneously at room temperature. Add 2-ethylfuran (105 μL, 1 mmol) to the well-stirred flask, react at room temperature for 24 h, and detect the reaction with a TLC plate until the reaction of the raw material is complete. A small amount of NaHCO was then added to the reaction mixture 3 Neutralize excess TFA, extract three times with ether, combine organic phases; wash with water, wash with saturated brine, wash with MgSO 4 Dry, concentrate under reduced pressure, and separate by column chromatography with n-hexane as the eluent to obtain self-coupling product IIa (76 mg, 80%).
[0017] IR (KBr) υ 3116, 2973, 2933, ...
Embodiment 2
[0019] IIb
[0020] In a 10mL single-necked flask was added Pd(OAc) 2 (22.4 mg, 10 mmol %), sealed and evacuated, replaced the air in the bottle with an oxygen balloon, and filled the flask with oxygen. Then, DMSO (1.5 mL), TFA (75 μL, 1 mmol) were sequentially added to the flask via syringe under an oxygen atmosphere, and the reaction mixture was stirred homogeneously at room temperature. Add 2-benzyloxymethylenefuran (188 mg, 1 mmol) to a well-stirred flask, react at 50°C for 24 hours, and detect the reaction with a TLC plate until the reaction of the raw materials is complete. A small amount of NaHCO was then added to the reaction mixture 3 Neutralize excess TFA, extract three times with ether, combine organic phases; wash with water, wash with saturated brine, wash with MgSO 4 Dry, concentrate under reduced pressure, and separate by column chromatography, using n-hexane / ethyl acetate as the eluent at 1:20, to obtain the self-coupling product IIb (159 mg, 85%).
[0...
Embodiment 3
[0023] IIc
[0024] In a 10mL single-necked flask was added Pd(OAc) 2 (22.4 mg, 10 mmol %), sealed and evacuated, replaced the air in the bottle with an oxygen balloon, and filled the flask with oxygen. Then, DMSO (1.5 mL), TFA (75 μL, 1 mmol) were sequentially added to the flask via syringe under an oxygen atmosphere, and the reaction mixture was stirred homogeneously at room temperature. Add ethyl 2-furan propionate (168 mg, 1 mmol) to the well-stirred flask, react at 50°C for 24 h, and detect the reaction with a TLC plate until the reaction of the raw materials is complete. A small amount of NaHCO was then added to the reaction mixture 3 Neutralize excess TFA, extract three times with ether, combine organic phases; wash with water, wash with saturated brine, wash with MgSO 4 Dry, concentrate under reduced pressure, and separate by column chromatography, using n-hexane / ethyl acetate as the eluent at 1:10, to obtain the self-coupling product IIc (136 mg, 81%).
[0025]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com