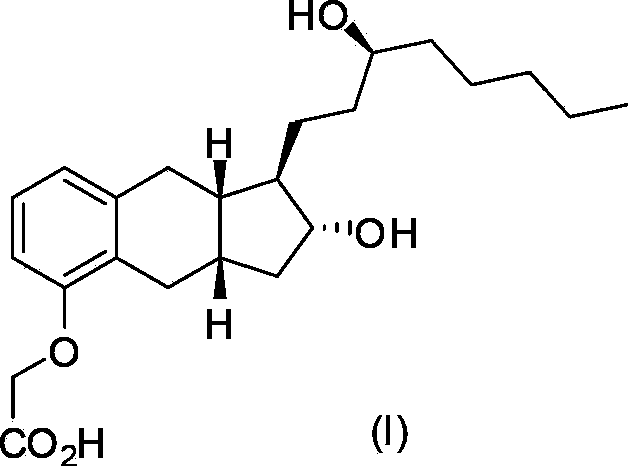
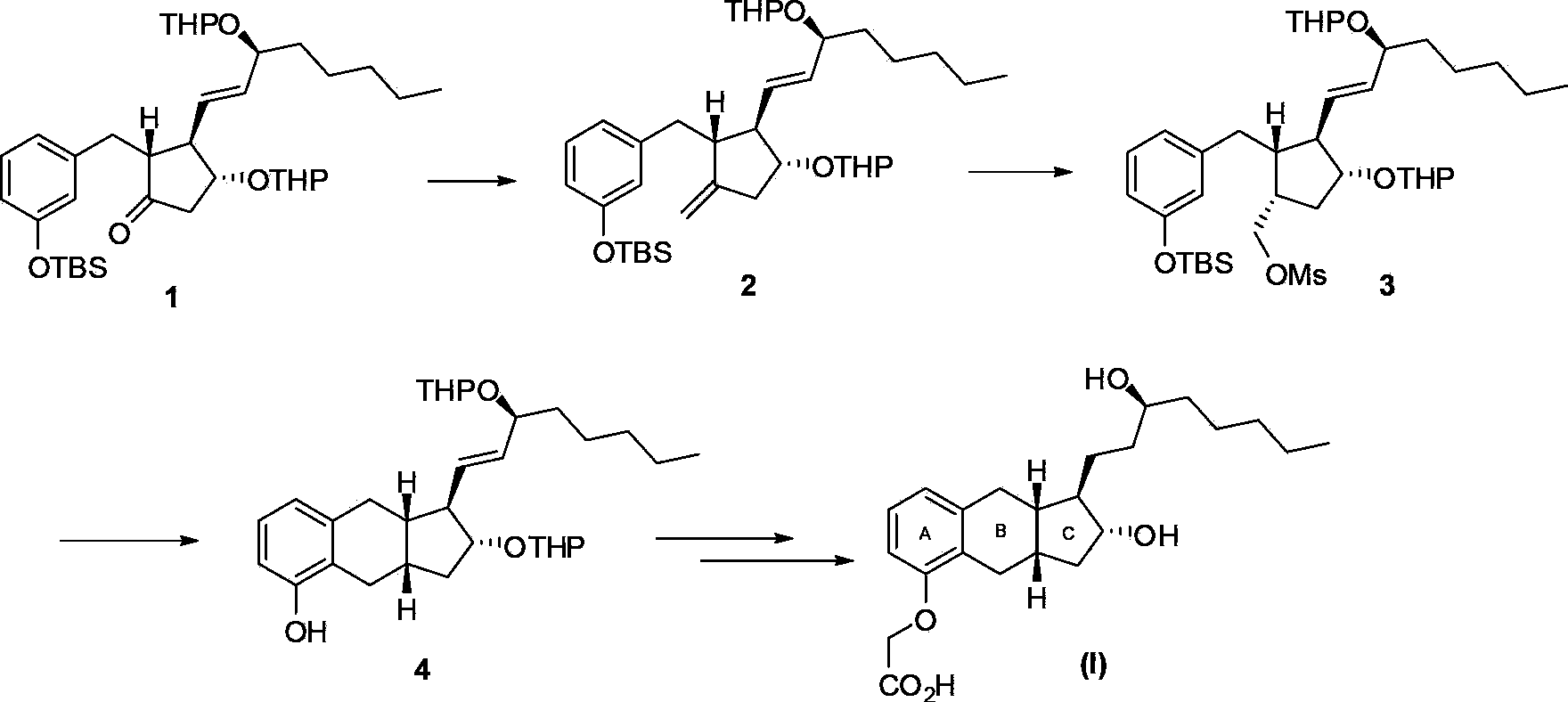
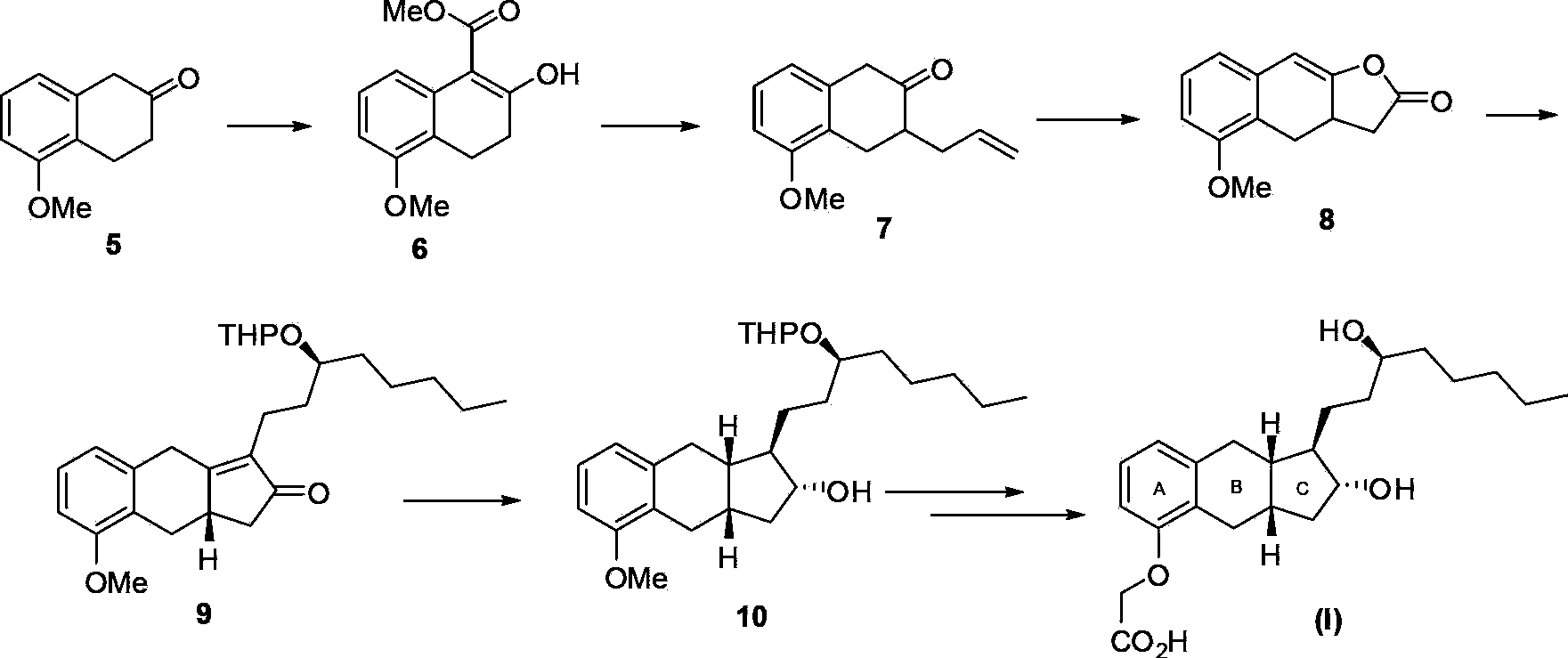
Intermediates for synthesizing treprostinil and preparation method thereof as well as the preparation method of treprostinil thereby
A compound and alkyl technology, applied in the field of intermediates for the preparation of Treprostinil, can solve the problems of excess and high synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: Preparation of compound Xa
[0081] Under nitrogen protection, 1-(trimethylsilyl) propyne (XVIa, purchased from Shanghai Ruiyi Pharmaceutical Technology Co., Ltd.) (74 g) in anhydrous tetrahydrofuran (200 ml) was added dropwise at 0°C. Lithium (250 mL, 2.5M in hexane). After stirring at 0°C for 3 hours, a solution of compound XIIa (65.8 g, synthesized with reference to literature: Tetrahedron2010, 66, 2351) in anhydrous tetrahydrofuran (100 ml) was added dropwise, and the reaction mixture was slowly warmed to 20°C and stirred at 20°C 12 hours. The reaction was quenched with saturated aqueous ammonium chloride, ethyl acetate and water were added to separate the layers, and the organic phase was collected. The organic phase was concentrated to obtain the crude product XIa as a yellow oil, which was directly used in the next reaction without purification.
[0082] To a solution of the crude product XIa in ethanol was slowly added sodium hydroxide (39.2 g) ...
Embodiment 2
[0084] Embodiment 2: preparation compound VIIIa
[0085] Under the protection of nitrogen, add a toluene solution of dimethyl zinc (200 ml, 1.0M) to a solution of compound Xa (47.3 g) in toluene (200 ml) at 20°C, and then add compound XIIIa (3.80 g, synthesized by referring to literature: Tetrahedron: Asymmetry2005, 16, 1953) in toluene solution. The mixture was cooled to -10°C, and a toluene solution of compound IXa (12.6 g, synthesized by reference to J. Am. Chem. Soc. 1985, 107, 1421) was added dropwise. After stirring at -10°C for 6 hours, the reaction was quenched with saturated aqueous ammonium chloride, ethyl acetate was added and water was separated, and the organic phase was collected. The organic phase was concentrated and separated by column chromatography to obtain compound VIIIa (23.2 g, yield 95%).
[0086] VIIIa: 1 HNMR (400MHz, CDCl 3 )δ7.46-7.16(m,7H),6.91(d,J=8.2Hz,1H),6.05-5.95(m,1H),5.87-5.71(m,1H),5.64(s,1H),5.08 (s, 2H), 5.05-4.89 (m, 4H), 4.70-4.60 ...
Embodiment 3
[0087] Embodiment 3: preparation compound VIIIa
[0088] step one:
[0089] To a solution of compound Xa (39 g) in anhydrous tetrahydrofuran (150 ml) was added dropwise n-butyllithium (61 ml, 2.5M hexane solution) at -78°C under nitrogen. After stirring at -78°C for 1 hour, a solution of compound IXa (30 g, synthesized by referring to literature: J. Am. Chem. Soc. 1985, 107, 1421.) in anhydrous tetrahydrofuran (100 ml) was added dropwise. After stirring at -78°C for 1 hour, it was quenched with water, ethyl acetate and water were added and the organic phase was collected. The organic phase was concentrated and separated by column chromatography to obtain compound XIVa (53.0 g, yield 91%).
[0090] XIVa: 1 H NMR (400MHz, CDCl 3 )δ7.46-7.16(m,7H),6.91(d,J=8.2Hz,1H),6.05-5.95(m,1H),5.87-5.71(m,1H),5.64(s,1H),5.08 (s, 2H), 5.05-4.89 (m, 4H), 4.70-4.60 (m, 1H), 3.90-3.42 (m, 5H), 2.50-1.27 (m, 16H).
[0091] Step two:
[0092] To a solution of compound XIVa (45.4 g) in anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com