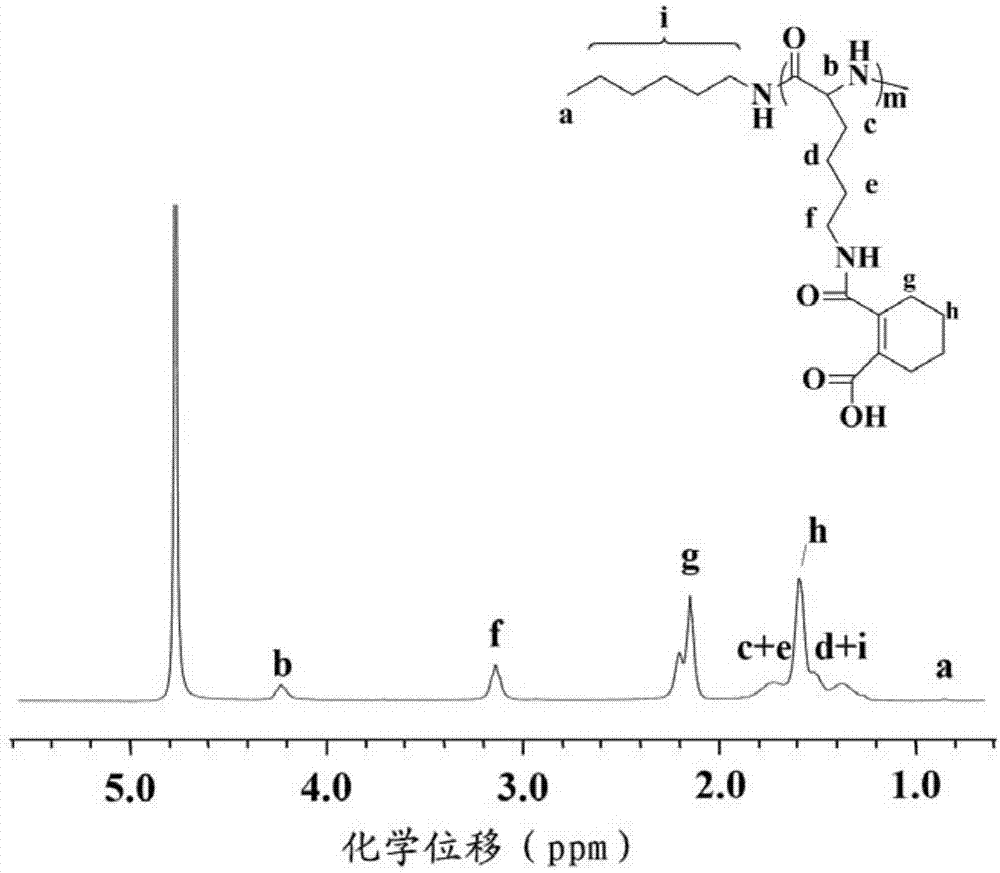
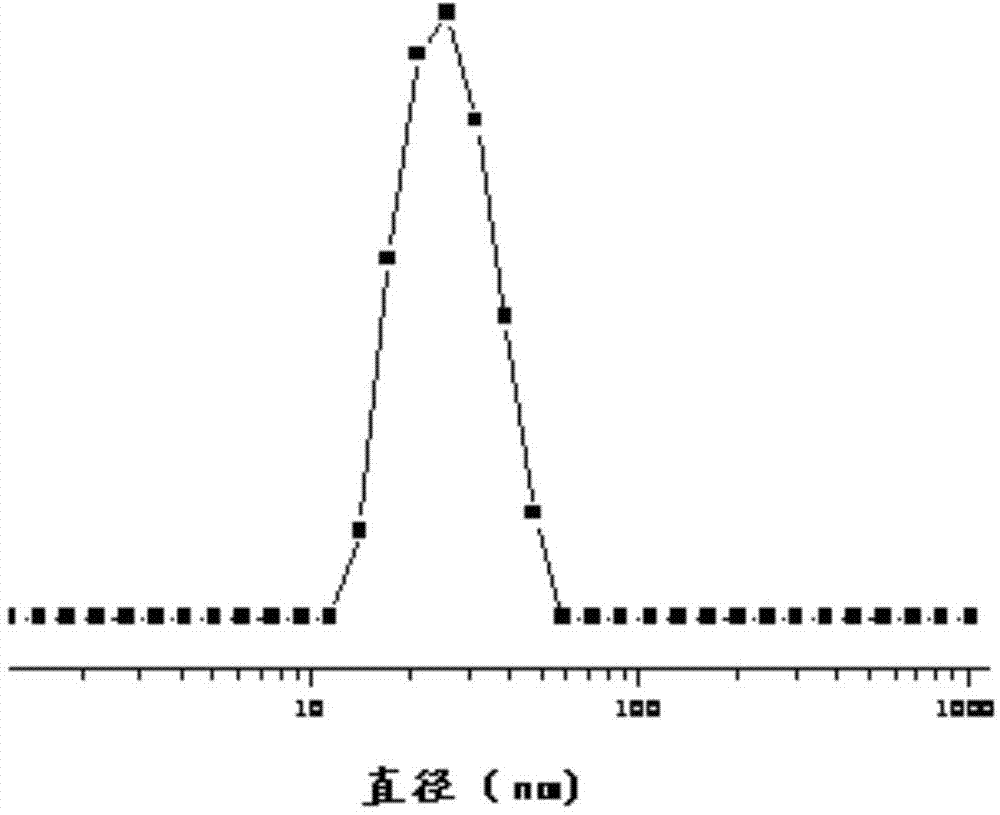
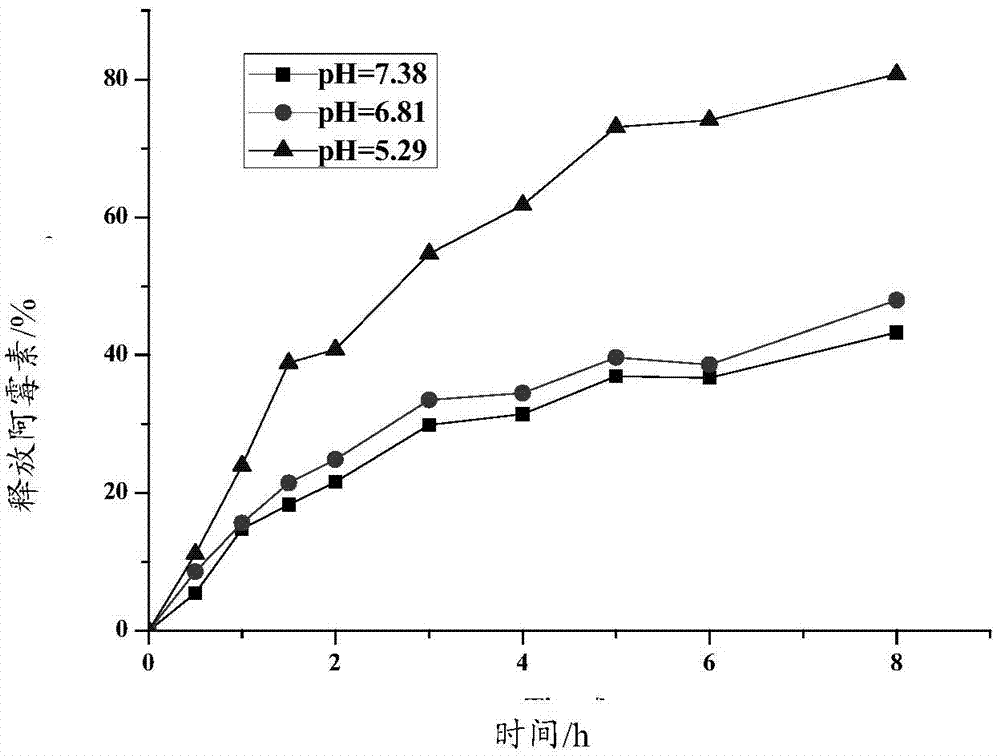
Responsive polymeric micelle drug carrying system and preparation method thereof
A polymer glue, responsive technology, used in pharmaceutical formulations, active ingredients of heavy metal compounds, anti-tumor drugs, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The present invention also provides a preparation method for the functionalized polyethylene glycol monomethyl ether-b-poly(L-lysine) represented by the above formula (I), comprising: A) the polyethylene glycol with terminal amination Monomethyl ether is mixed with N(ε)-benzyloxycarbonyl-L-lysine-N-cyclic acid anhydride in an organic solvent to react to obtain N-benzyloxycarbonyl-protected polyethylene glycol monomethyl ether-b -poly(L-lysine); B) deprotecting the N-benzyloxycarbonyl-protected polyethylene glycol monomethyl ether-b-poly(L-lysine) in a strongly acidic solution to obtain Polyethylene glycol monomethyl ether-b-poly(L-lysine); C) Mix the polyethylene glycol monomethyl ether-b-poly(L-lysine) with acid anhydride for reaction, dialyze, Freeze-dry to obtain functionalized polyethylene glycol monomethyl ether-b-poly(L-lysine); the acid anhydride is succinic anhydride, 2,3,4,5-tetrahydrophthalic anhydride, dimethylmaleic One or more of acid anhydride, tetramethy...
Embodiment 1~5
[0081] Weigh 10g of polyethylene glycol monomethyl ether with a number average molecular weight of 1000 (0.01mol), 2000 (0.005mol), 5000 (0.002mol), 10000 (0.001mol) and 20000 (0.0005mol), respectively, and put them into 5 In a dry reaction flask with a branched mouth, add 100ml of toluene to azeotropically remove water, then dissolve the obtained solids in 100ml of anhydrous dichloromethane, cool to 0°C, add 5.06g (0.05mol) , 2.53g (0.025mol), 1.01g (0.010mol), 0.51g (0.005mol) and 0.25g (0.0025mol) triethylamine, and then dropwise added 22.91g, 11.46g, 4.58g, 2.29g and 1.15g Methanesulfonyl chloride. After the addition of methanesulfonyl chloride, react at 0°C for 2h, return to 25°C, and continue to react for 24h under stirring. After the reaction is completed, filter to remove the formed precipitate. The filtrate is settled with ether, filtered, washed, and vacuum-dried at 25°C for 24h , to obtain polyethylene glycol monomethyl ether methanesulfonate. Carry out nuclear ma...
Embodiment 6
[0086] Mix 1mol N-benzyloxycarbonyl-L-lysine and 0.6mol bis(trichloromethyl)carbonate at 25°C, add tetrahydrofuran, heat to 50°C for 2 hours, after the reaction, put the reaction mixture in Settled in excess petroleum ether, separated, washed, recrystallized and dried to obtain N(ε)-benzyloxycarbonyl-L-lysine-N-cyclic acid anhydride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com