Methods for producing recombinant adenovirus and drug preparations of recombinant adenovirus with serum-free suspension cells
A technology of recombinant adenovirus and suspension cells, applied in biochemical equipment and methods, viruses/bacteriophages, microorganisms, etc., can solve the problems that cannot meet the requirements of large-scale industrialization of gene therapy products, and achieve the effect of large-scale industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
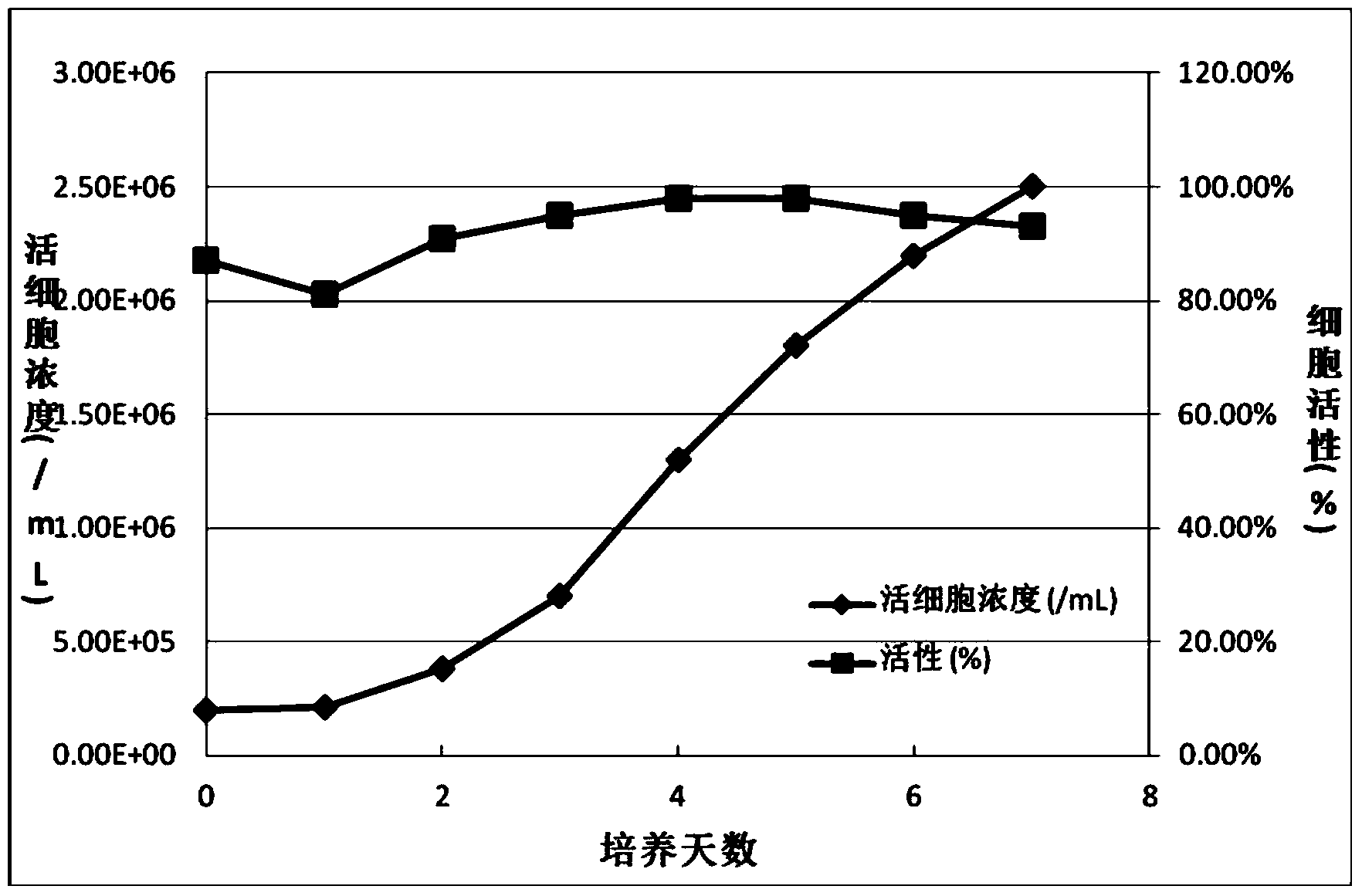
[0048] Example 2, 293F cells (#11625-019) were purchased from Invitrogen in the United States. 293F cells were cultured and expanded in suspension in spinner bottles with serum-free CD293 medium. After obtaining a certain amount of cells, the cells are frozen. The cell freezing liquid adopts CD293+10%DMSO, the cell concentration is 1-5x10 7 / ml. Cells were frozen using a programmable cell freezer. Then store it in the upper vapor layer of the liquid nitrogen tank for later use.
[0049]In order to ensure the purity and stability of the cells, the cells were cloned and screened by the terminal dilution method. The resulting cell clone was named SBN-293SFS cells. SBN-293SFS cells were suspended and expanded in spinner bottles with serum-free CD293 medium. After obtaining a certain amount of cells, the cells are frozen. The cell freezing liquid adopts CD293+10%DMSO, the cell concentration is 1-5x10 7 / ml. Cells were frozen using a programmable cell freezer. Then store i...
Embodiment 3
[0057] Example 3: Large-scale high-density culture of SBN-293SFS cells and infection of recombinant adenovirus using Wave-20 perfusion bioreactor.
[0058] In order to avoid the complexity of centrifuging a large amount of cell culture medium under sterile conditions, we adopted a method of diluting high-density SBN-293SFS cells with fresh CD293 medium, and the dilution factor was about ten times. Provide enough fresh medium for the cells. We used the Wave-20 perfusion bioreactor to culture SBN-293SFS cells at high density on a large scale. Cell concentration can reach or exceed 1x10 7 / ml. When the cell concentration reaches 1x10 7 / ml, add fresh CD293 medium to dilute the cells to 1x10 6 / ml, and at the same time transfer the cell culture medium to another bioreactor with larger volume for the infection and production of recombinant adenovirus.
[0059] Thaw a tube of SBN-293SFS cells and inoculate into a spinner bottle with 100 ml of CD293 medium. At 37°C, 10% CO 2 C...
Embodiment 4
[0064] Example 4: Large-scale high-density culture of SBN-293SFS cells and infection and production of 100 liters of recombinant adenovirus and separation and purification by ion exchange column chromatography using Wave-20 perfusion bioreactor.
[0065] After confirming the feasibility of large-scale high-density culture of SBN-293SFS cells and diluted cells for recombinant adenovirus infection in Wave-20 perfusion bioreactor, all cells cultured in Wave-20 perfusion bioreactor were treated with recombinant adenovirus. The virus was infected with a total volume of 100 liters. The multiplicity of infection was 50 virus particles / cell. The pH of the bioreactor is controlled between 6.5-8.2, the DO is controlled between 15%-75%, the temperature of the culture medium is controlled between 36-37°C, the swing speed is controlled between 15-20rpm, and the swing angle is controlled between Between 8-15 degrees. After culturing for 4 days, Tween-20 was added to the cell culture mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com