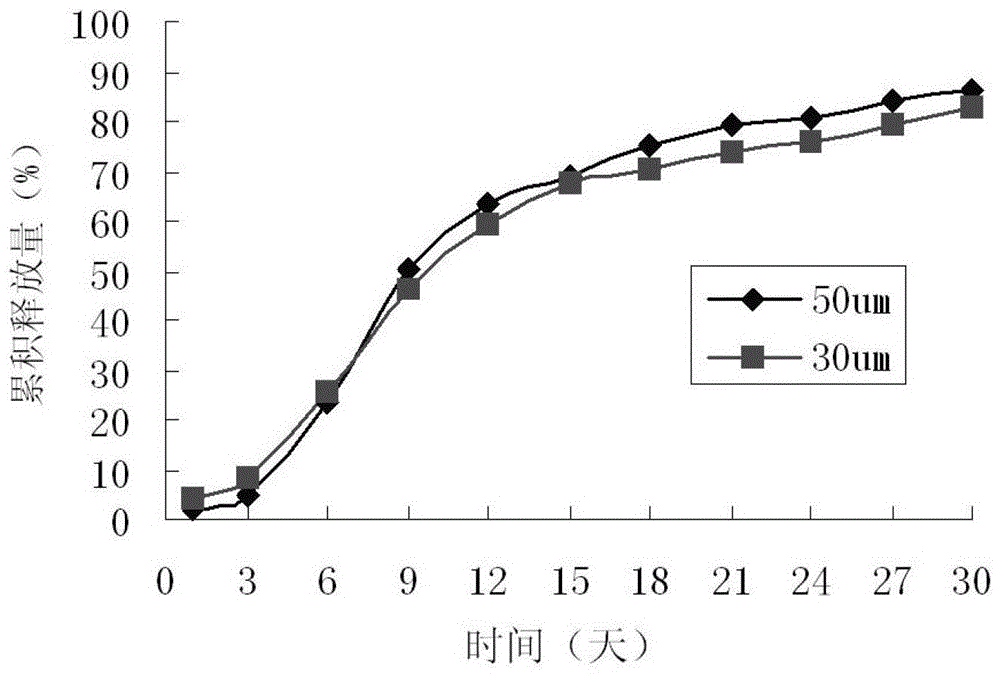
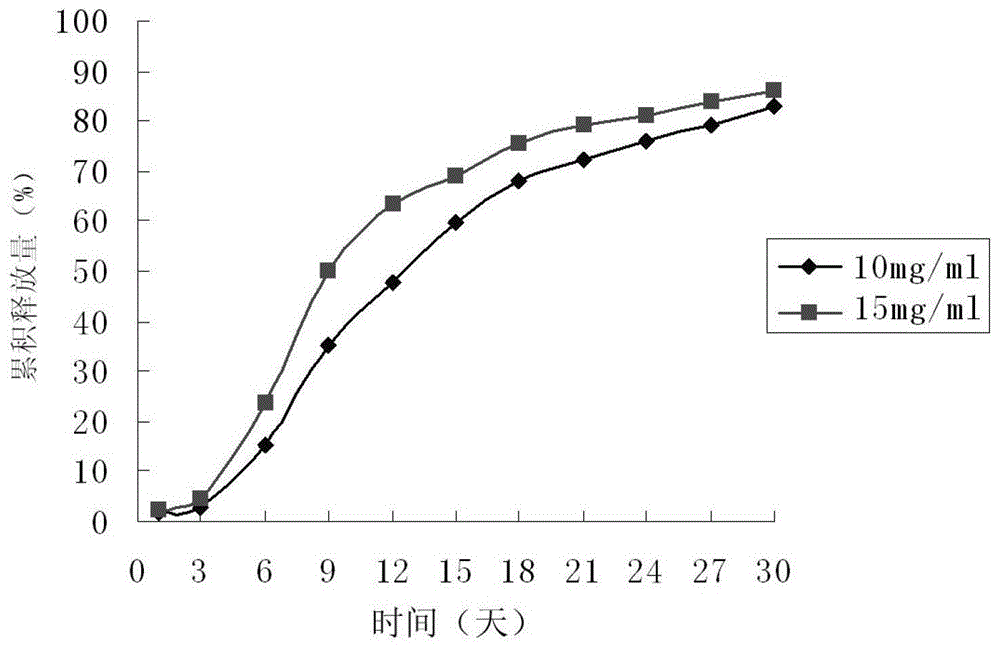
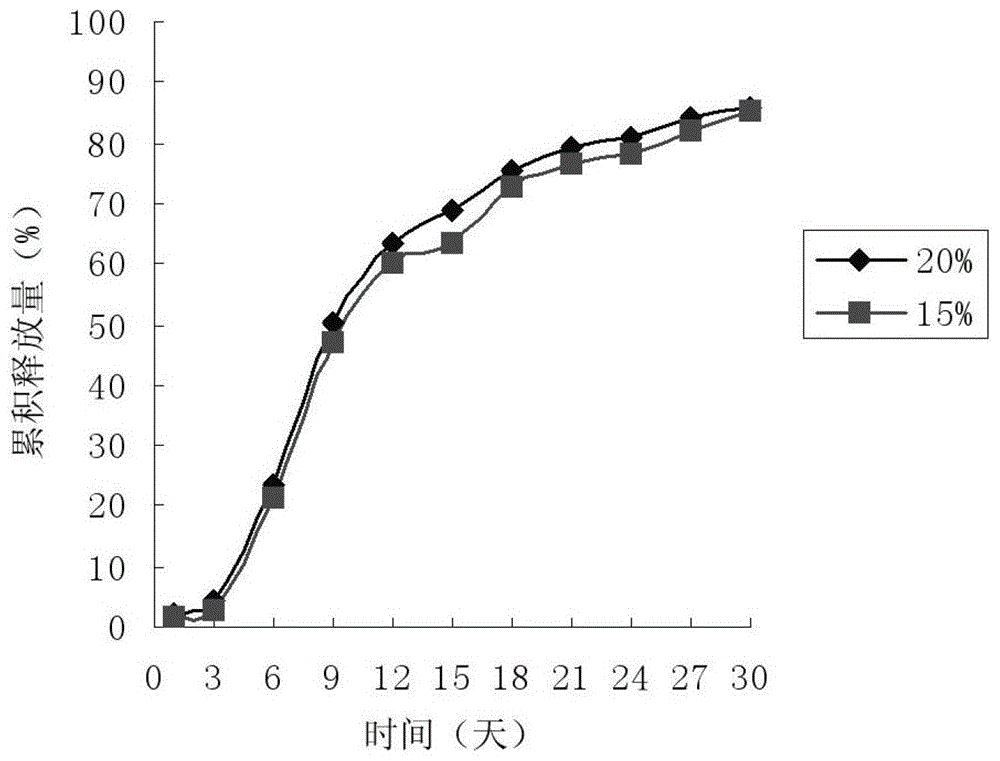
Paliperidone sustained-release microspheres, injection thereof and preparation method of the sustained-release microspheres
A technology of paliperidone and slow-release microspheres, which is applied to medical preparations containing non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. Side effects, increased metabolic by-products and other issues, to achieve the effect of improving bioavailability, relieving pain, and high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The third aspect of the present invention provides a method for preparing paliperidone sustained-release microspheres, the method comprising the following steps:
[0047] 1) Provide an organic solution of paliperidone and polylactic-co-glycolic acid; and provide an aqueous solution of an emulsifier;
[0048] 2) adding the organic solution to the aqueous solution for emulsification; and
[0049] 3) Separation to obtain the organic phase containing the microspheres, and remove the organic solvent therein to prepare paliperidone sustained-release microspheres;
[0050] Wherein, the volume ratio of the organic solution to the aqueous solution is (7-9):(100-120), preferably 7:100.
[0051] Preferably, the organic solvent in the organic solution is selected from dichloromethane, ethyl acetate or a mixture thereof.
[0052] Preferably, in the organic solution, the polylactic acid-glycolic acid copolymer has a concentration of 0.0085-0.0100 g / ml, and the paliperidone has a co...
Embodiment 1
[0075] Experimental steps:
[0076] 1. Weigh an appropriate amount of PVA, add water to dissolve at 90°C, allow to cool to room temperature, constant volume, and prepare a 0.03g / ml PVA solution to obtain an aqueous phase solution.
[0077] 2. Take 1000ml of the aqueous phase solution obtained in step 1 and place it in a Fluko reaction vessel for later use.
[0078] 3. Weigh 0.175g of Paliperidone bulk drug and 0.7g of PLGA, add 70ml of dichloromethane to dissolve it completely, and obtain an organic phase solution, that is, in this organic phase solution, the concentration of Paliperidone bulk drug is 0.0025 g / ml, the concentration of PLGA is 0.01g / ml.
[0079] 4. Add the organic phase solution dropwise to the aqueous phase solution in the Fluko reaction vessel with a separatory funnel, and perform mechanical stirring while adding dropwise at a stirring speed of 60rmp; centrifuge after stirring for 6 hours, discard the upper layer solution, and collect the lower layer of micr...
Embodiment 2
[0083] Experimental steps:
[0084] 1. Weigh an appropriate amount of PVA, add water to dissolve at 95°C, let it cool to room temperature, constant volume, and prepare a 0.03g / ml PVA solution to obtain an aqueous phase solution.
[0085] 2. Take 1000ml of the aqueous phase solution obtained in step 1 and place it in a Fluko reaction vessel for later use.
[0086] 3. Weigh 0.185 g of paliperidone bulk drug and 0.8 g of PLGA, add 80 ml of dichloromethane to dissolve them completely, and obtain an organic phase solution.
[0087] 4. Add the organic phase solution dropwise to the aqueous phase solution in the Fluko reaction vessel with a separatory funnel, mechanically stir while adding dropwise, the stirring speed is 60rmp, centrifuge after stirring for 7 hours, discard the upper layer solution, collect the lower layer of microspheres, The obtained microspheres were washed three times with water, collected, subpackaged and freeze-dried. The freeze-drying method was freezing at -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com