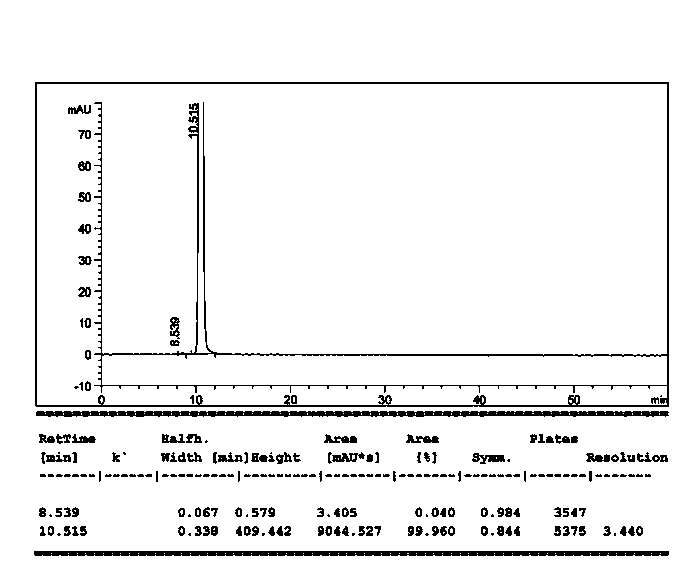
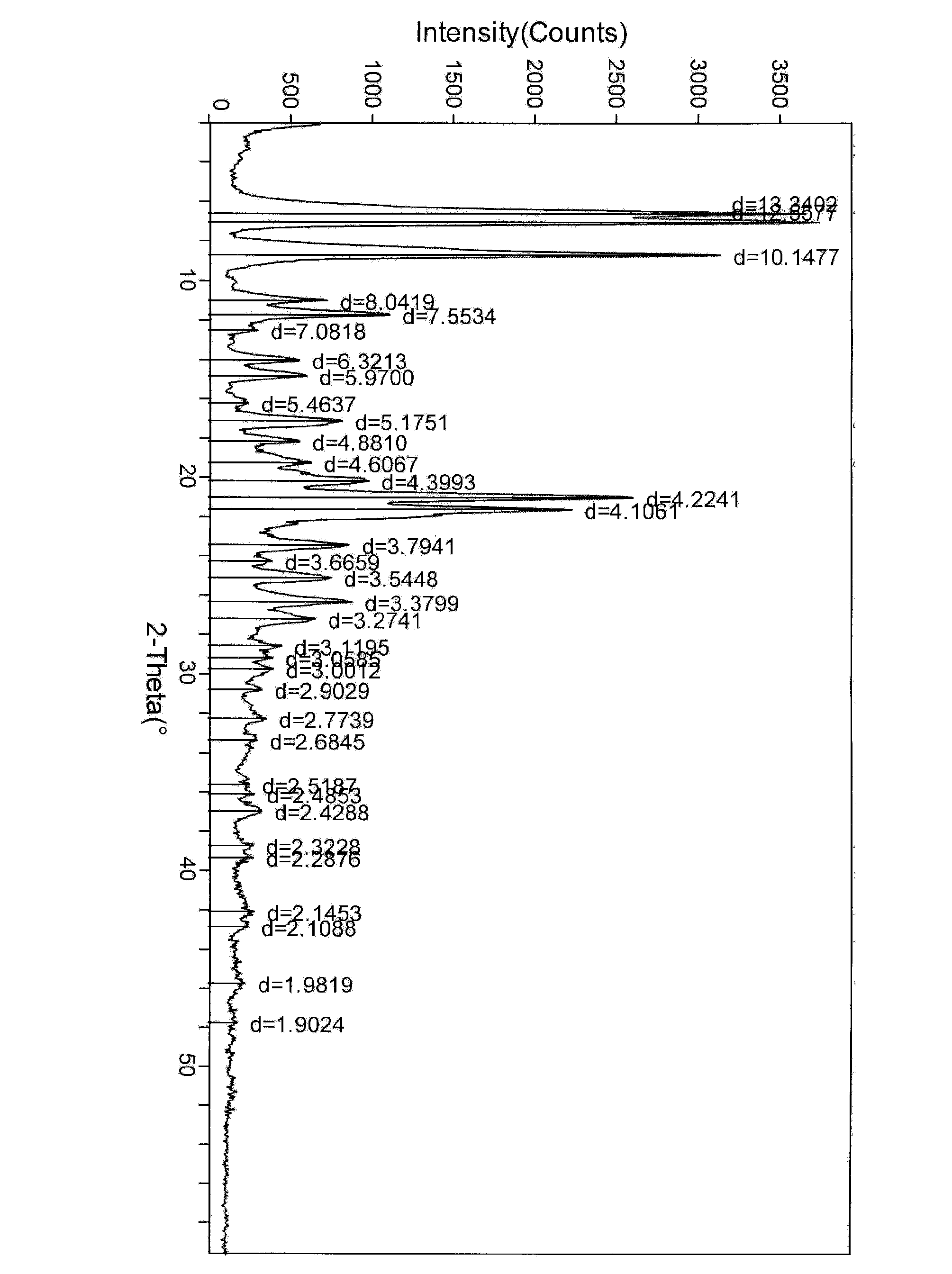
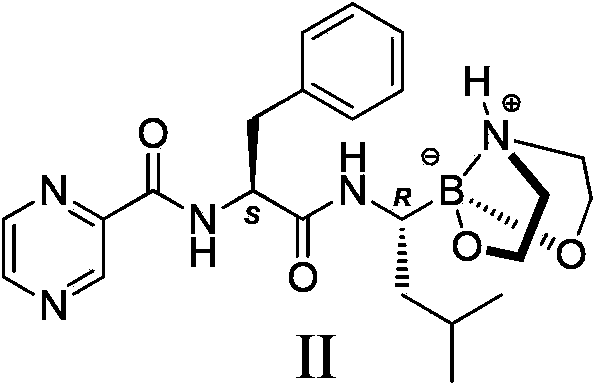
Crystal form of bortezomib key intermediate, and preparation method and application of crystal form
A technology of bortezomib and crystal form, which is applied in the field of high-purity bortezomib API products, can solve the problems of no research, no study of crystallization conditions, no provision of crystal form and crystal preparation method, etc., to achieve stable properties, Ease of storage and transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] Dissolve 4.6 grams of compound Ia (prepared according to the document J.Am.Chem.Soc.2008, 130, 6910-6911) in ethyl acetate, add 2.6 grams of diethanolamine, and stir at room temperature for 12 hours, that is, a large amount of solids precipitate out , filtered to obtain the crude product of Compound II, and washed the filter cake with ethyl acetate to obtain 4.0 g of α-form crystal product of Compound II (yield 88.4%).
Embodiment 2
[0053]
[0054] Dissolve 5.2 grams of compound Ib (prepared according to patent CN200580017645) in methanol, add 2.6 grams of diethanolamine, stir at room temperature for 24 hours, a large amount of solids precipitate out, filter to obtain the crude compound II, wash the filter cake with methanol to obtain 4.4 grams The α-form crystal product of compound II (yield 97.3%).
Embodiment 3
[0056]
[0057] Dissolve 4.5 g of compound Ic (prepared according to patent CN201210147057) in tert-butyl methyl ether, add 2.6 g of diethanolamine, stir at room temperature for 24 hours, a large amount of solids precipitate out, filter to obtain the crude compound II, and wash the filter cake with tert-butyl methyl ether That is, 4.2 g of α-form crystal product of compound II was obtained (yield 92.9%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com