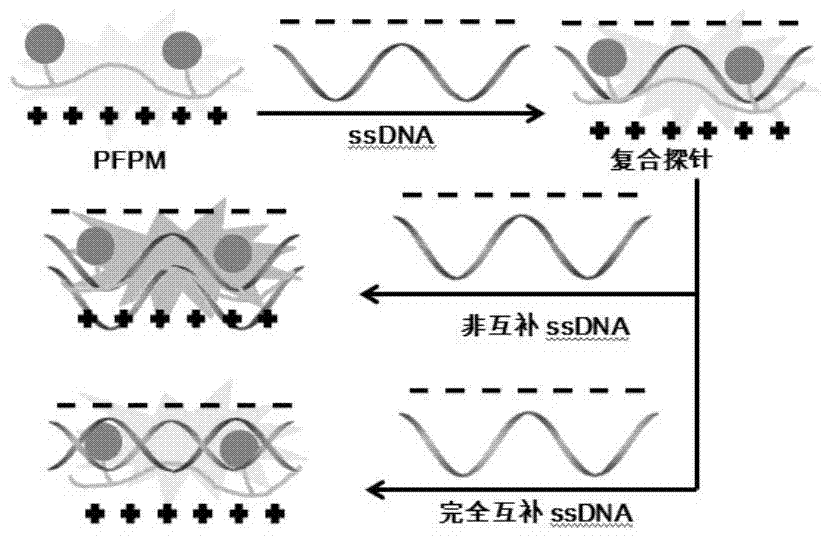
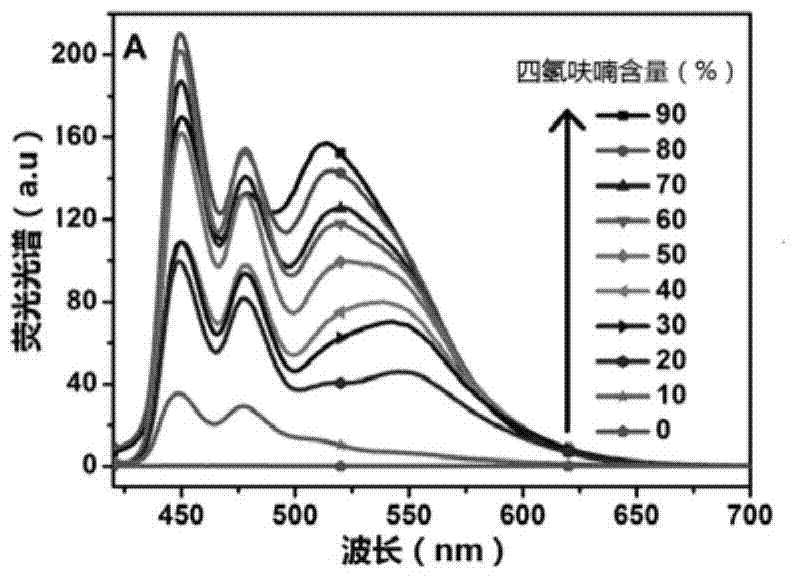
Perylene grafted poly (4-vinylpyridine), synthesis thereof and preparation of fluorescent probe
A vinylpyridine, fluorescent probe technology, applied in the synthesis of fluorescent polyelectrolyte, fluorescent probe field, can solve the problem of not high fluorescence energy resonance transfer efficiency, affecting product fluorescence efficiency and test results, etc., to achieve product fluorescence energy resonance Effects of improved transfer efficiency and improved fluorescence efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
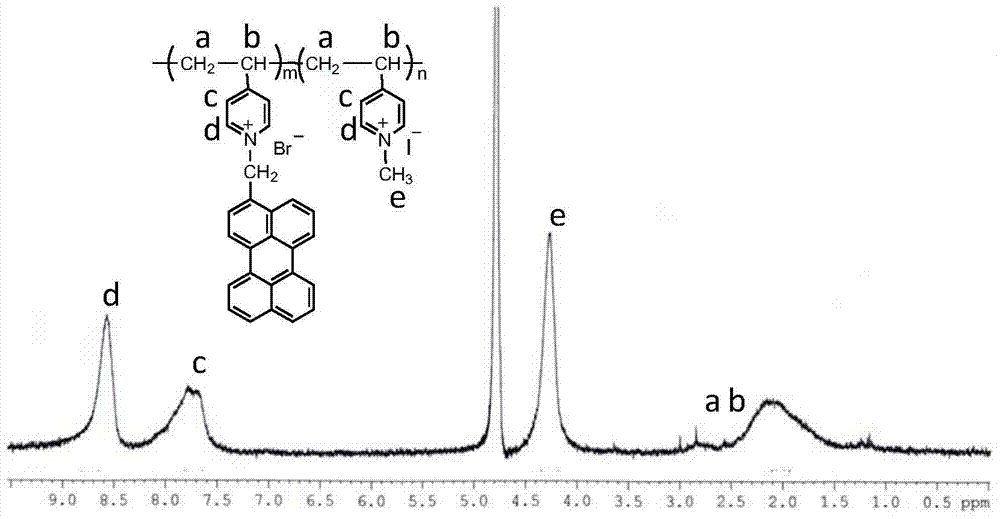
[0030] Synthesis of a perylene-grafted poly(4-vinylpyridine): Add 5 mmol of poly(4-vinylpyridine) with a weight-average molecular weight of 60,000 into a three-necked flask, add 15 mL of chloroform to dissolve, and then add 0.2mmol of perylene bromide, under nitrogen protection, condensed and refluxed at 68°C for about 24 hours. After the reaction, the reaction solution was slowly added dropwise into a large amount of tetrahydrofuran to obtain a precipitate, which was repeatedly dissolved and precipitated, and then vacuum-dried to obtain PFP. Dissolve PFP in an appropriate amount of absolute ethanol, add a large amount of iodomethane, protect with nitrogen, and stir and reflux at 40°C for 24 hours. After the reaction is finished, the reaction solution is vacuum rotary distilled to obtain a solid powder product, which is further vacuum dried to obtain the final product PFPM. The grafting rate of perylene in the finally measured PFPM is 2.5%, and the weight average molecular we...
Embodiment 2
[0038]Synthesis of a perylene-grafted poly(4-vinylpyridine): Add 5 mmol of poly(4-vinylpyridine) with a weight-average molecular weight of 60,000 into a three-necked flask, add 15 mL of chloroform to dissolve, and then add 0.2mmol of perylene bromide, under nitrogen protection, condensed and refluxed at 68°C for about 24 hours. After the reaction, the reaction solution was slowly added dropwise into a large amount of tetrahydrofuran to obtain a precipitate, which was repeatedly dissolved and precipitated, and then vacuum-dried to obtain PFP. Dissolve PFP in an appropriate amount of absolute ethanol, add a large amount of iodomethane, protect with nitrogen, and stir and reflux at 40°C for 24 hours. After the reaction is finished, the reaction solution is vacuum rotary distilled to obtain a solid powder product, which is further vacuum dried to obtain the final product PFPM. The grafting rate of perylene in the finally measured PFPM is 2.5%, and the weight average molecular wei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com