Preparation method of high-performance lithium ion battery negative material Mn2OBO3
A lithium-ion battery and negative electrode material technology, applied in battery electrodes, nanotechnology for materials and surface science, secondary batteries, etc., can solve the problem of granular products without morphology, poor cycle stability, long reaction time, etc. problem, achieve the effect of short reaction time, low reaction temperature and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Mn 2 OBO 3 Preparation of nanorod materials
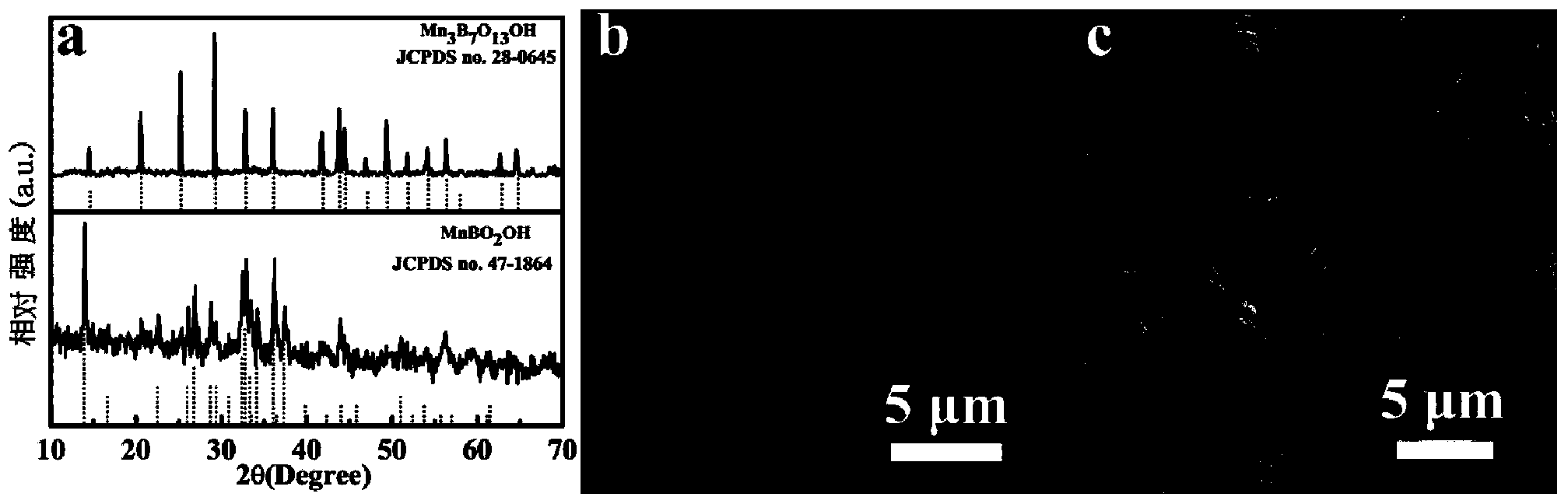
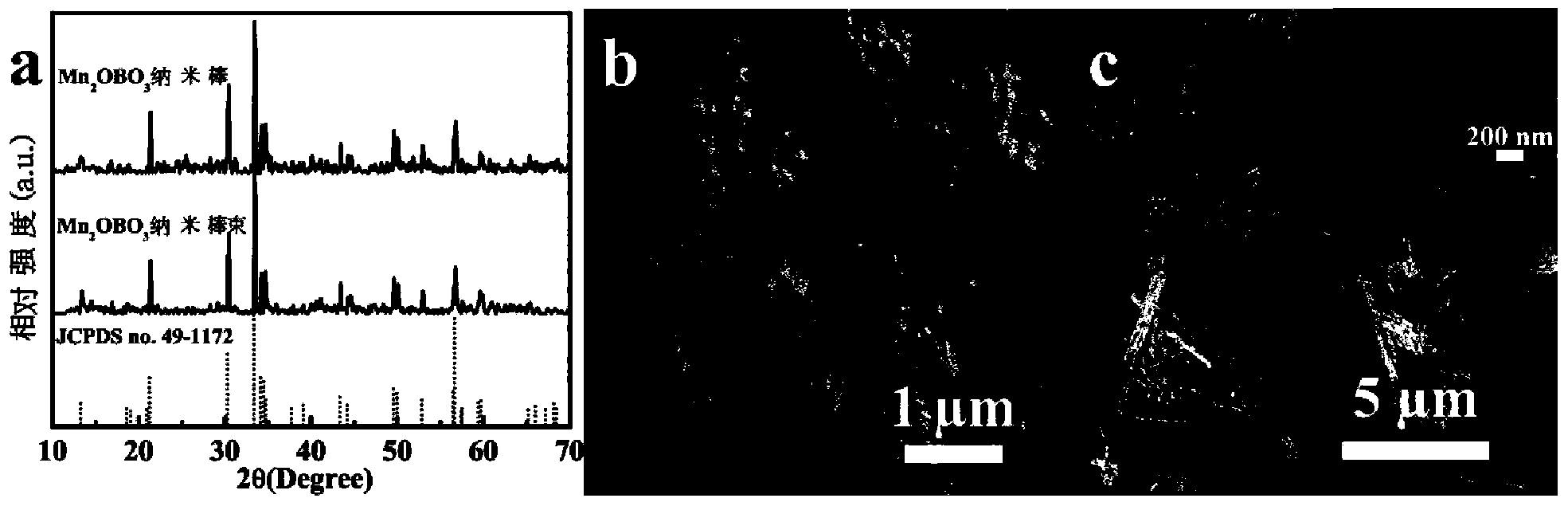
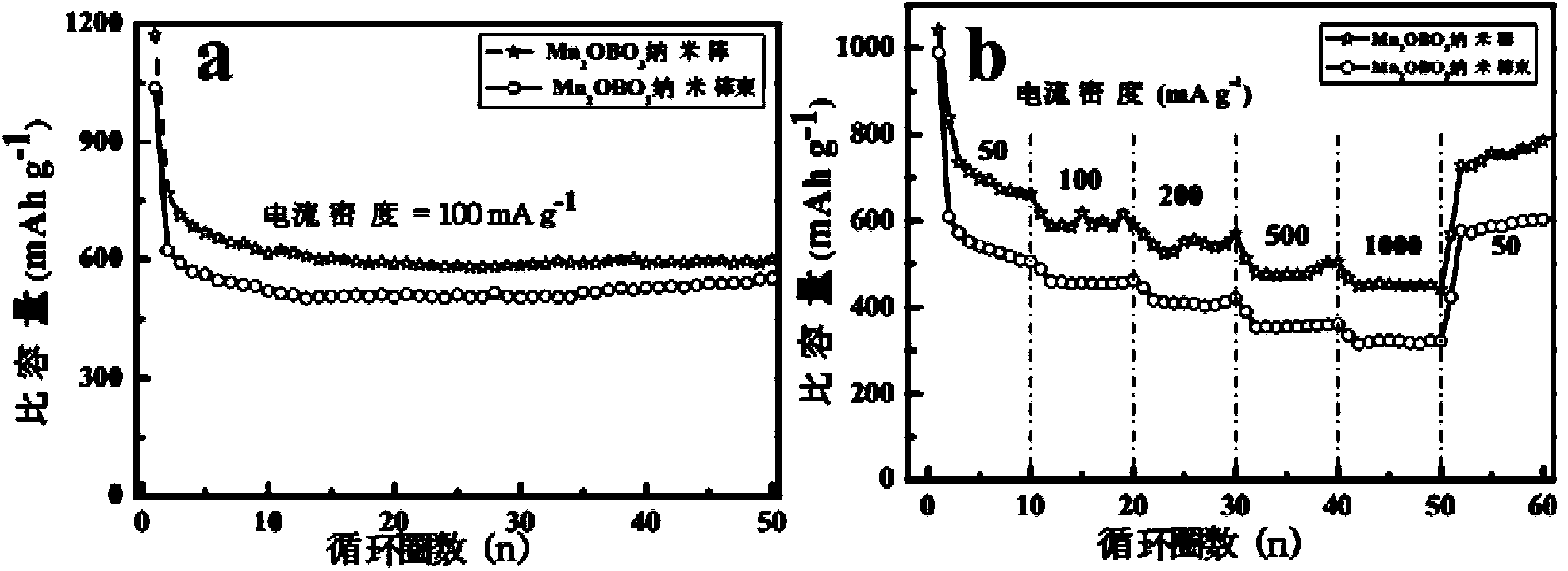
[0046] 0.01mol NH 4 HB 4 O 7 ﹒ 3H 2 O was dissolved in 20 mL of deionized water, and then 0.01 mol of Mn (NO 3 ) 2 The aqueous solution was dropped into the above solution, and finally 20 mL of deionized water was added. After fully stirring and mixing for 1 hour, it was transferred to a 60 mL polytetrafluoroethylene kettle, sealed with a stainless steel kettle shell, and then placed in an oven at 220 ° C to react for 24 hours, and cooled. After reaching room temperature, carry out suction filtration to obtain Mn 3 B 7 O 13 OH nanorod precursor. Mn 3 B 7 O 13 The OH nanorod precursor was calcined at 750 °C for 12 hours in a resistance furnace in an air atmosphere to obtain Mn 2 OBO 3 Nano stave. Obtained Mn 3 B 7 O 13 OH nanorod precursor and final product Mn 2 OBO 3 The XRD and SEM results of the nanorods are as follows: figure 1 a. figure 1 b. figure 2 a. figure 2 b, from figure 1 a. figure 2...
Embodiment 2
[0048] Mn 2 OBO 3 Preparation of Nanorod Bundle Materials
[0049] 0.01mol NH 4 HB 4 O 7 ﹒ 3H 2 O was dissolved in 20 mL of ethanol, and then 0.01 mol of Mn (NO 3 ) 2 The solution was dropped into the above solution, and finally 20 mL of ethanol was added. After fully stirring and mixing for 1 hour, it was transferred to a 60 mL polytetrafluoroethylene kettle, sealed with a stainless steel kettle shell, and then placed in an oven at 220 ° C for 12 hours, and cooled to room temperature. Then carry out suction filtration to obtain MnBO 2 OH nanorod bundle precursor. MnBO 2 Mn was obtained by calcining the precursor of OH nanorod bundles at 700 °C for 5 hours in a resistance furnace in an air atmosphere. 2 OBO 3 Bundle of nanorods. The resulting MnBO 2 OH nanorod bundle precursor and final product Mn 2 OBO 3 The XRD and SEM results of nanorod bundles are as follows: figure 1 a. figure 1 c. figure 2 a. figure 2 c, from figure 1 a. figure 2 It can be seen f...
Embodiment 3
[0053] Other morphology Mn 2 OBO 3 material preparation
[0054] 0.01mol NH 4 HB 4 O 7 ﹒ 3H 2 O was dissolved in 20 mL of tetraethylene glycol, and then 0.01 mol of Mn (NO 3 ) 2 The aqueous solution was dropped into the above solution, and finally 20 mL of tetraethylene glycol was added. After fully stirring and mixing for 1 hour, it was transferred to a 60 mL polytetrafluoroethylene kettle, sealed with a stainless steel kettle shell, and then placed in an oven at 220 ° C to react for 24 hours, and cooled. After reaching room temperature, centrifugal washing is performed to obtain Mn 3 B 7 O 13 OH triangular-shaped precursor. Mn 3 B 7 O 13 The OH triangular-shaped precursor was calcined at 700 °C for 5 hours in a resistance furnace in an air atmosphere to obtain triangular-shaped Mn 2 OBO 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com