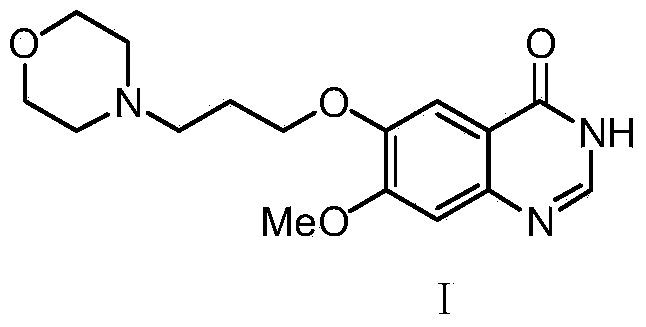
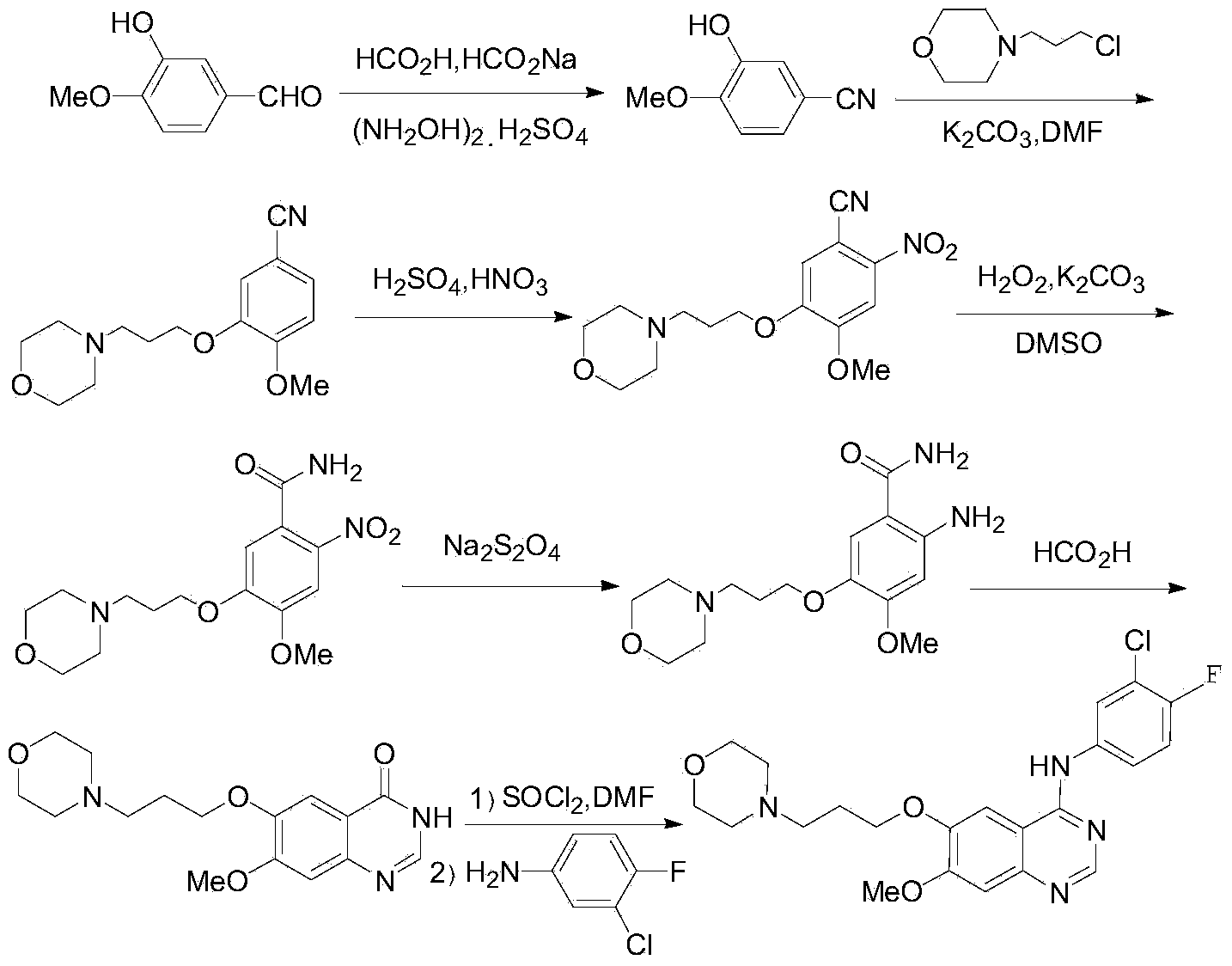
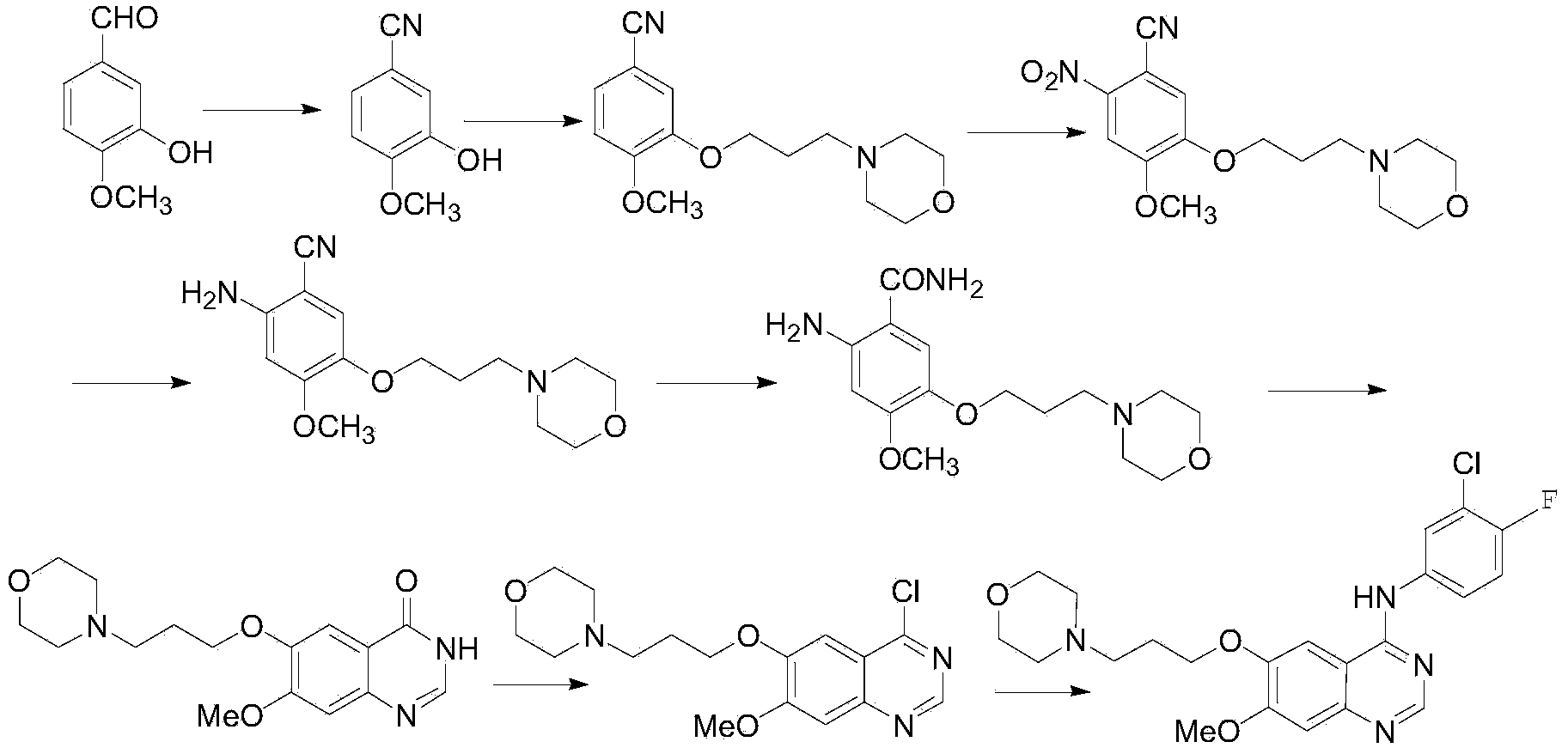
Method for preparing 7-methoxy-6-(3-morpholine-4-propoxy) quinazoline-4(3H)-ketone
A technology of methoxyquinazoline and base propoxy, applied in the field of organic compound synthesis, can solve the problems of complicated operation, excessively long process route steps, unfavorable for industrialized production, etc. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The compound represented by formula II: 6-hydroxy-7-methoxyquinazolin-4-one (19.2g, 100mmol) was slowly added to 140g of 12% KOH aqueous solution, stirred and reacted for 2.0 hours until dissolved into a transparent solution , part of the water was removed under reduced pressure (until a large amount of solid precipitated, the same below), cooled, filtered, and the solid was dried to obtain the dipotassium salt of the compound represented by formula III (25.8 g, yield 96.3%).
[0041] Disperse the dipotassium salt of the compound shown in formula III (10.7g, 40mmol) in 20mLDMF, then add 5% [Bmim]BF4 ionic liquid of the dipotassium salt of the compound shown in III, add the compound shown in formula IV (X =Cl) (7.9g, 48mmol), heated to about 55-60°C for 5.0 hours, the reaction mixture was suction filtered while it was hot, and the white solid KCl formed was removed, the solvent DMF was evaporated, and 20mL of distilled water was added, stirred at room temperature and adde...
Embodiment 2
[0043] Slowly add the compound shown in formula II (11.5g, 60mmol) into 60g of 12% NaOH aqueous solution, stir and react for 2 hours, remove part of the solvent under reduced pressure, cool, filter, and dry the disodium of the compound shown in formula III Salt (13.2 g, yield 93.2%).
[0044] Disperse the disodium salt of the compound shown in formula III (7.1g, 30mmol) in 20mLTHF, then add 5% [Bmim]BF of the disodium salt of the compound shown in III 4 Ionic liquid, add compound (X=Br) (7.5g, 36mmol) shown in formula IV, heat to about 65-70°C and react for 5.0 hours, the reaction mixture is filtered while hot to remove the formed solid NaBr, and the solvent THF is evaporated Add 20mL of distilled water, stir at room temperature and add 0.5M hydrochloric acid solution to adjust the pH value to 6-7, remove excess compound shown in IV by steam distillation, suction filter, and dry the solid to obtain the compound shown in formula I (9.0g, yield 93.8%).
Embodiment 3
[0046] Slowly add the compound shown in formula II (11.5g, 60mmol) into 84g of 12% KOH aqueous solution, stir and react for 2 hours, remove part of the solvent under reduced pressure, cool, filter, and dry the dipotassium compound shown in formula III Salt (15.3 g, yield 95.1%).
[0047]Disperse the disodium salt (13.4g, 50mmol) of the compound shown in formula III in 40mL DMF, then add 5% [Bmim] BF4 ionic liquid of the dipotassium salt quality of the compound shown in III, add the compound shown in formula IV (X =I) (15.3g, 60mmol), heated to about 75-80°C and reacted for 5.0 hours, the reaction mixture was suction filtered while it was hot, the white solid KI was removed, the solvent DMF was evaporated, and 20mL of distilled water was added, stirred at room temperature and added 0.5 M hydrochloric acid solution to adjust the pH value to 6-7, steam distillation to remove excess compound shown in IV, suction filtration, and solid drying to obtain compound shown in formula I (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com