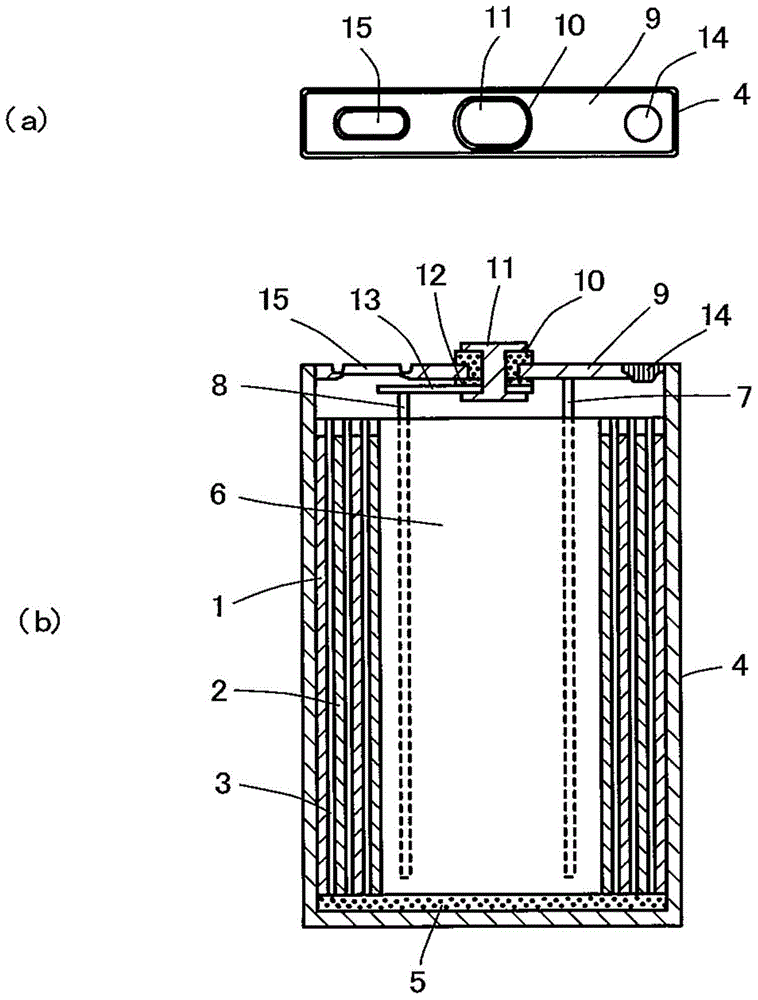
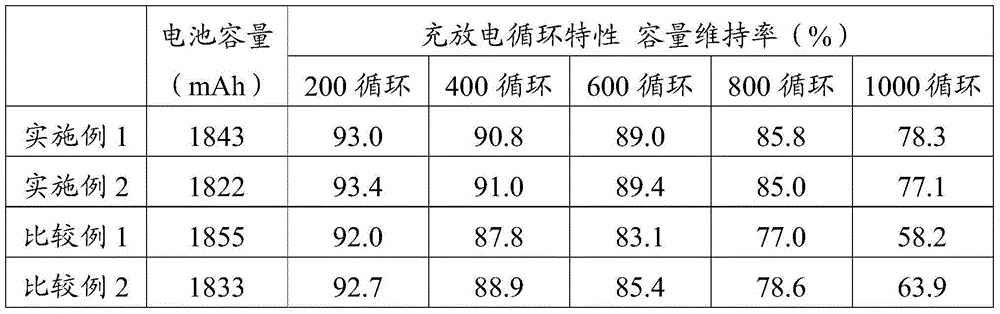
Non-aqueous electrolyte second battery
A non-aqueous electrolyte, secondary battery technology, applied in non-aqueous electrolyte batteries, secondary batteries, battery electrodes, etc., can solve the problems of capacity increase, battery tank expansion, gas generation, etc., and achieve good charge-discharge cycle characteristics, The effect of suppressing swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] (Synthesis of Lithium Composite Oxide)
[0126] Prepared respectively at 3.78mol / dm 3 , 0.25mol / dm 3 , 0.08mol / dm 3 , 0.08mol / dm 3 The concentration contains a mixed aqueous solution of nickel sulfate, cobalt sulfate, manganese sulfate and magnesium sulfate. Next, add ammonia water adjusted to a pH of about 12 by adding sodium hydroxide into the reaction vessel, and stir it vigorously. 3 / min, 6.6cm 3 The above mixed aqueous solution and 25% by mass ammonia water were added dropwise thereto at a rate of 1 / min to synthesize a coprecipitate compound (spherical coprecipitate compound) of Ni, Co, Mn, and Mg. And, at this time, the temperature of the reaction solution was maintained at 50°C, and 3mol / dm 3 The dropwise addition of concentrated sodium hydroxide aqueous solution keeps the pH of the reaction solution at about 12, and then the pH of the reaction solution is maintained at 1dm 3 Nitrogen blowing at a flow rate of / min.
[0127] The co-precipitated compound ...
Embodiment 2
[0150] (Synthesis of lithium nickel cobalt manganese composite oxide)
[0151] Aqueous ammonia adjusted to a pH of about 12 by adding sodium hydroxide is put into the reaction vessel, and it is vigorously stirred while using a quantitative pump at a rate of 23 cm 3 / min, 6.6cm 3 The following mixed aqueous solution and ammonia water of 25 mass % concentration are added dropwise thereto at a ratio of 1 / min to synthesize a coprecipitate compound (spherical coprecipitate compound) of Ni, Co and Mn, and the mixed aqueous solution is 2.0 mol / dm respectively 3 , 0.8mol / dm 3 , 1.2mol / dm 3 The concentration contains a mixed aqueous solution of nickel sulfate, cobalt sulfate and manganese sulfate. And, at this time, the temperature of the reaction solution is maintained at 50°C, and at the same time, the 6.4mol / dm 3 The dropwise addition of concentrated sodium hydroxide aqueous solution keeps the pH of the reaction solution at about 12, and then the pH of the reaction solution is m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com