Compositions and methods for the treatment of cancer
一种化合物、卤化物的技术,应用在化学仪器和方法、药物组合、铂类有机化合物等方向,能够解决限制应用等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
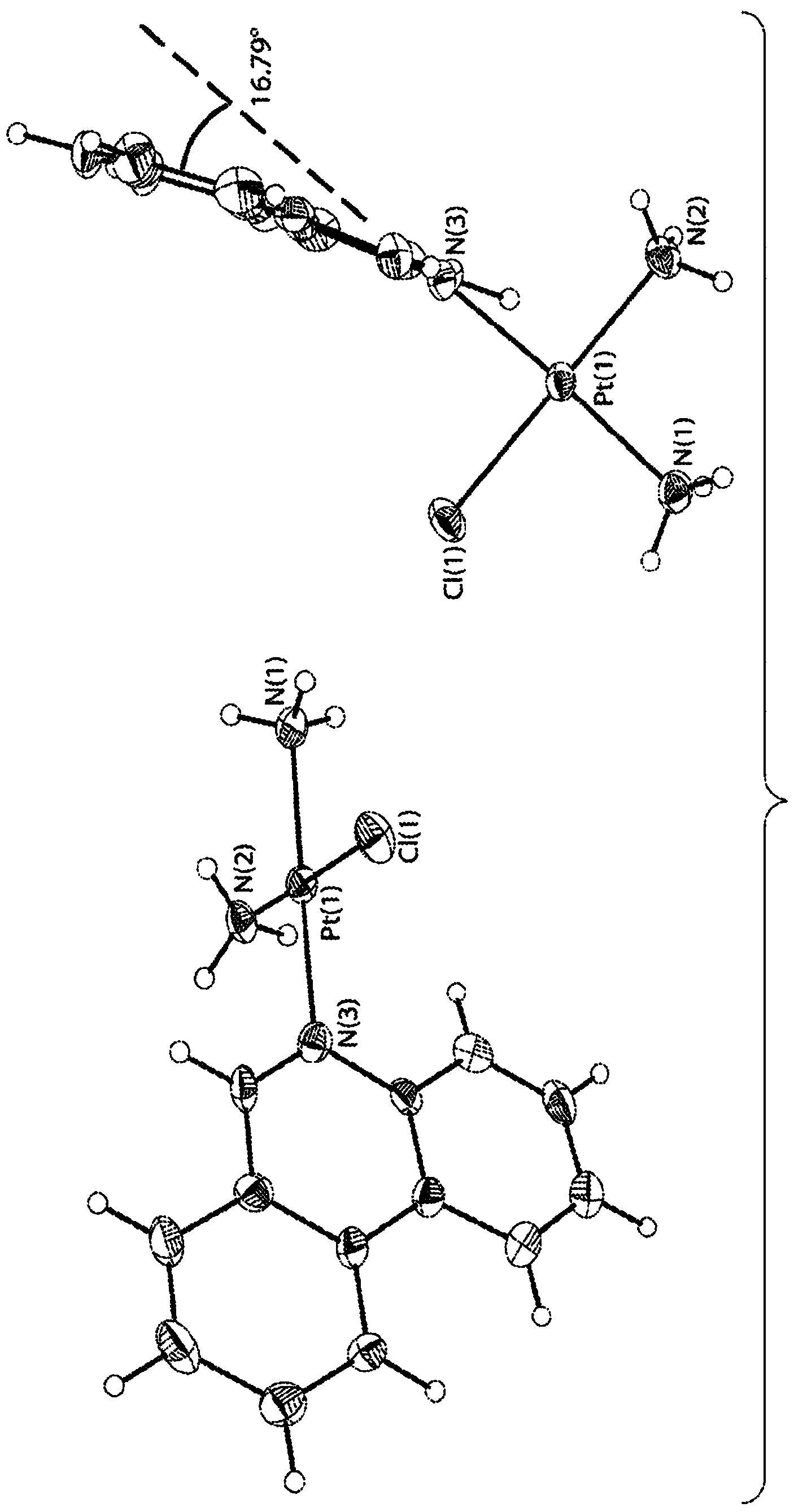
[0202] This example describes the synthesis and use of phenanthroplatin (eg, a compound of formula (VII)).
[0203] Experiment Details
[0204] materials and measurements. Picoplatin was synthesized as previously reported (J. Med. Chem. 1989, 32, 128-136). All other chemicals and solvents are commercially available. Recorded on a Bruker AVANCE-400 NMR spectrometer with a spectrally rotating superconducting magnet at the Massachusetts Institute of Technology Department of Chemistry Instrumentation Facility (MIT DCIF) 1 H. 13 C and 195 Pt NMR spectrum. Chemical shift for 195 Pt NMR reference in D 2 K in O 2 PtCl 4 (δ=-1628ppm) or for 1 H and 13 C NMR referenced to residual solvent peak. ESI-MS spectra were acquired on an Agilent Technologies 1100 Series LC / MS instrument. Atomic absorption spectroscopy was performed on a Perkin Elmer AAnalyst300 spectrometer. Distilled water was purified by passing through a Milli-Q Biocel water purification system (18.2 MΩ) with a ...
example 2
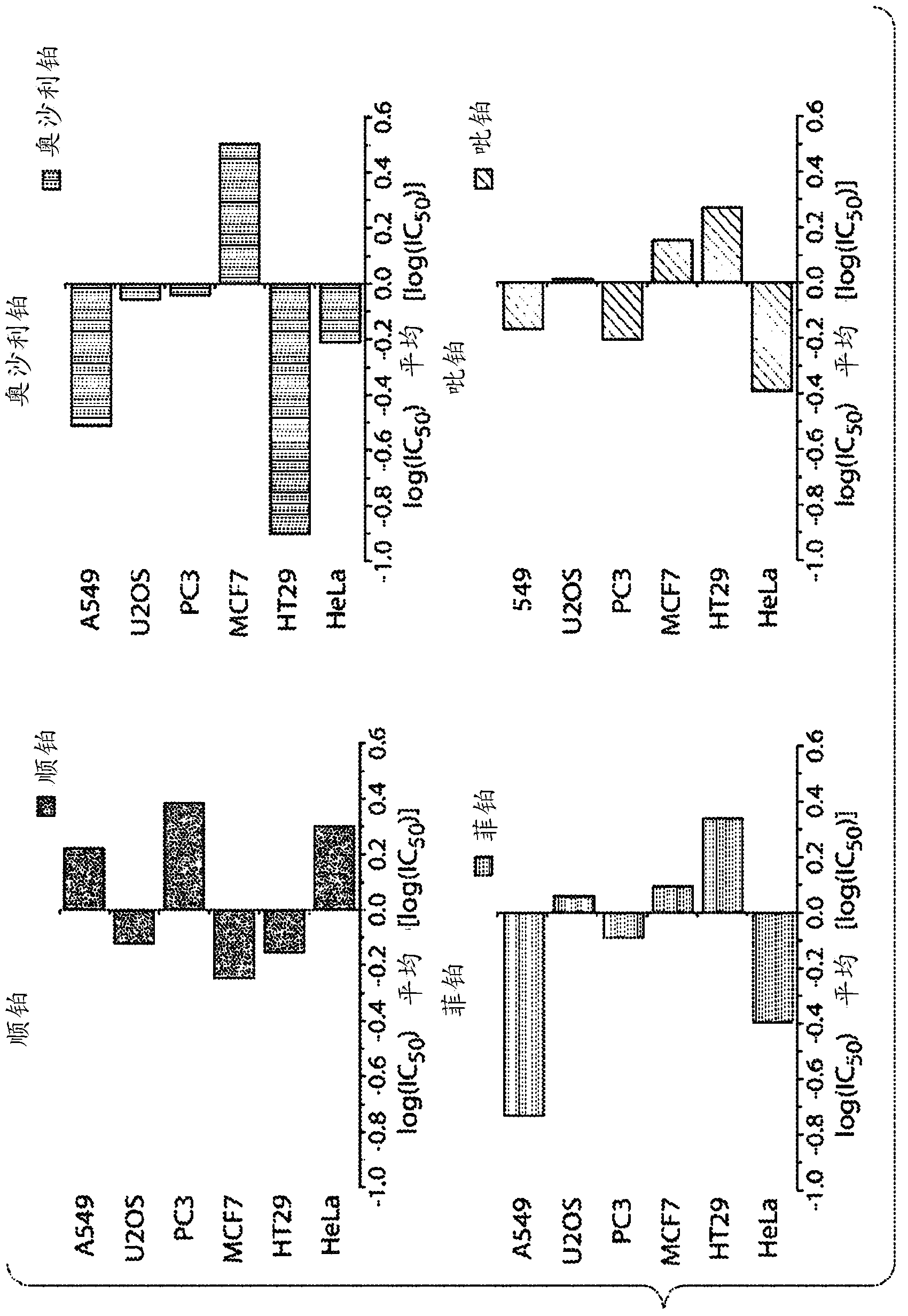
[0251] This example concerns the determination of the maximum tolerated dose (MTD) of phenanthroplatin in mice. The maximum tolerated dose (MTD) is defined as the highest dose of a drug or treatment that does not cause any adverse side effects. This example describes the determination of the MTD of phenoplatin administered intravenously to albino ICR mice.
[0252] Experimental part
[0253] animal. In this study, 42 healthy albino ICR female mice (19.9–28.7 g weight) were used.
[0254]Detailed process. In Phase 1 of the study, 12 ICR albino mice were assigned to receive 4 different concentrations of phenanthplatin via tail vein injection (Table 7). Three additional mice were dosed with PBS as vehicle controls. In Phase 2 of the study, 24 ICR albino mice were assigned to receive 8 different concentrations of phenanthplatin via tail vein injection (Table 8). Three additional mice were dosed with PBS as vehicle controls. Mice were observed for side effects immediately af...
example 3
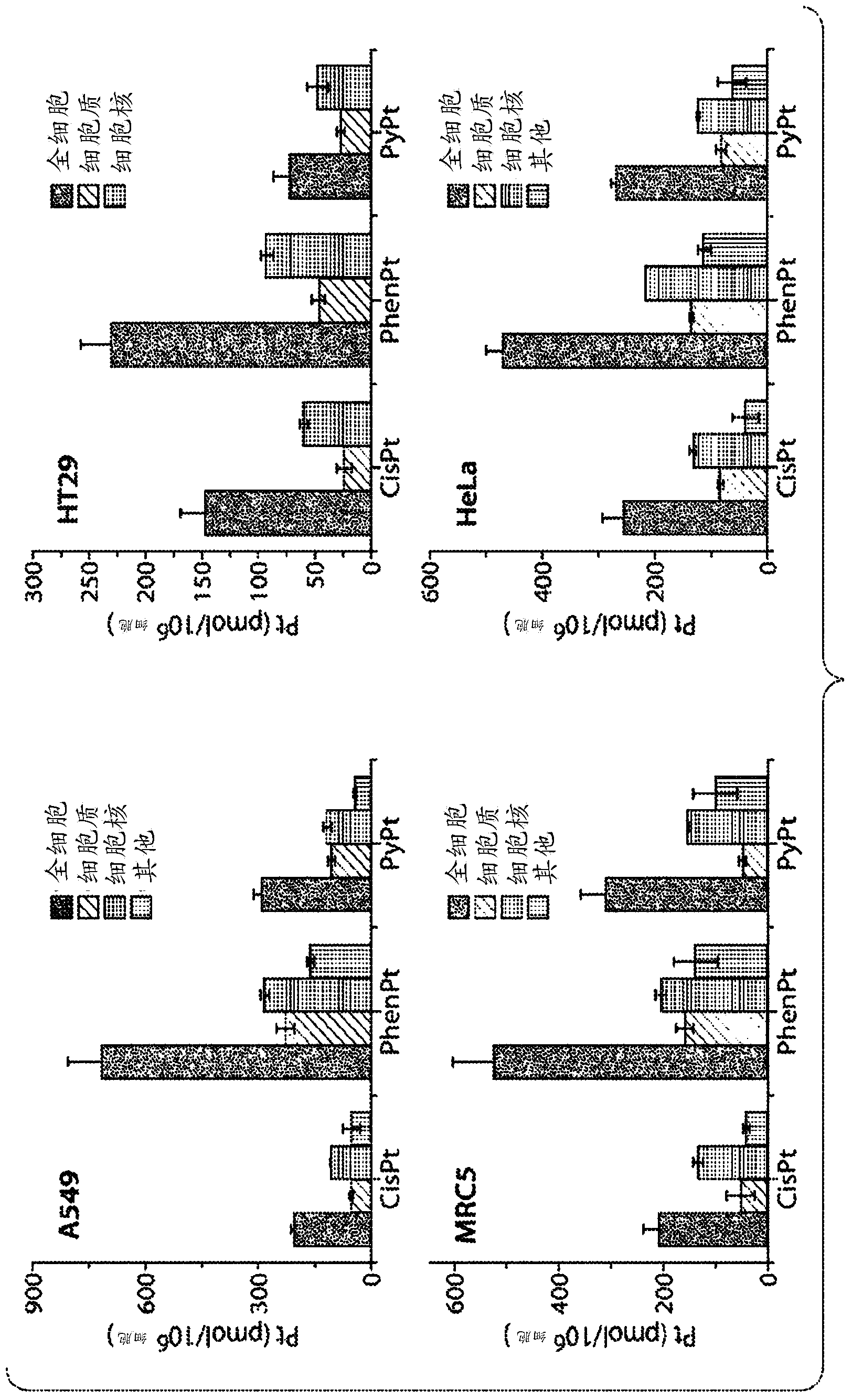
[0269] This example describes the encapsulation of phenanthrene platinum into polymeric nanoparticles by using the double emulsion method with PLGA-PEG-COOH and the nanoprecipitation method with functionalized PLA-OH and PLGA-PEG-COOH.
[0270] Experimental part
[0271] Materials and Measurements. Synthesis of phenanthroplatin was performed as previously described. All chemicals and solvents are commercially available. Recorded on a Bruker AVANCE-400 NMR spectrometer with a spectrally rotating superconducting magnet at the Massachusetts Institute of Technology Department of Chemistry Instrumentation Facility (MIT DCIF) 1 H. 13 C and 195 Pt NMR spectra. Electrospray ionization-MS (ESI-MS) spectra were acquired on an Agilent Technologies 1100 Series LC / MS instrument. Atomic absorption spectroscopy was performed on a Perkin Elmer AAnalyst600 spectrometer. Elemental analyzes were performed by Midwest Microlab, LLC, Indianapolis, IN. Distilled water was purified by passing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com