Application of thiol-polyethylene glycol in preparation of water-soluble gold nano-clusters
A thiol polyethylene glycol, gold nanocluster technology, applied in the field of nanomaterials, can solve the problems of unstable gold nanoclusters, poor modifiability, complex synthesis, etc., and achieve the effects of improving stability, good stability and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation method of water-soluble gold nanocluster comprises the following steps:
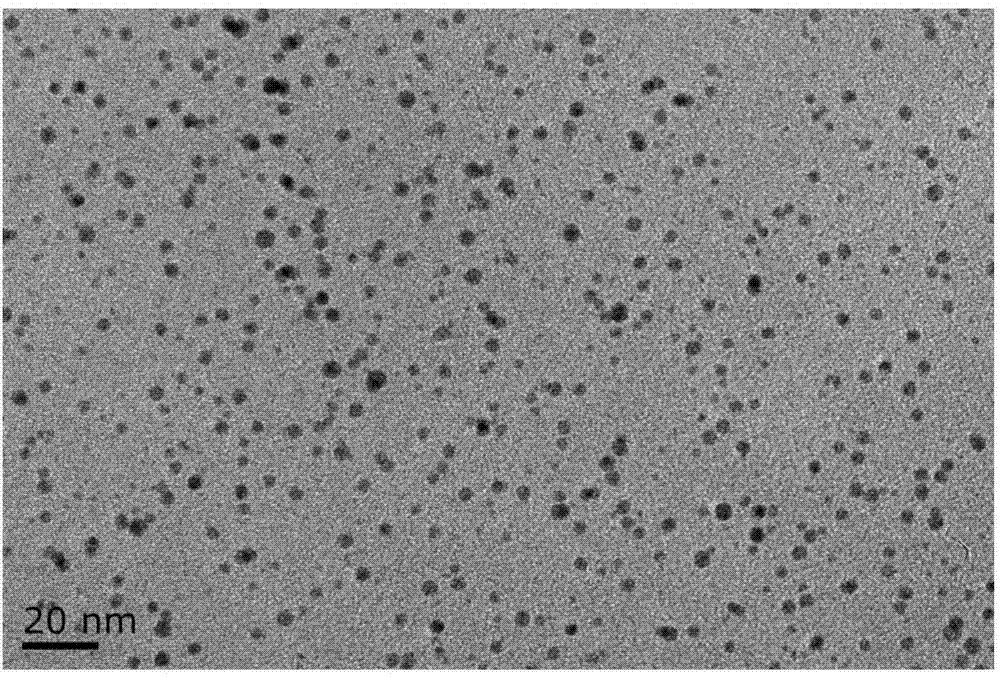
[0029] (1) Chlorauric acid and reducing agent were reacted in the dark for 3 hours at room temperature, and a yellow gold nanocluster precursor solution with blue-green fluorescence but unstable was obtained. The TEM results are shown in figure 1 .
[0030] (2) reacting the gold nanocluster precursor and mercapto polyethylene glycol at room temperature in the dark for 8 hours to obtain a light yellow gold nanocluster solution that emits orange-yellow fluorescence;
[0031] (3) Use a dialysis bag with a molecular weight of 2000 to dialyze the gold nanocluster solution to obtain a pure orange-yellow fluorescent colorless gold nanocluster solution. The TEM results are shown in figure 2 .
[0032] Wherein, the reducing agent is histidine, and the mercapto polyethylene glycol is HS-C 11 -EG 6 ; The molar ratio of chloroauric acid, reducing agent and mercapto polyethylene glycol is ...
Embodiment 2
[0036] The preparation method of water-soluble gold nanocluster comprises the following steps:
[0037] (1) Chlorauric acid and reducing agent were mixed and reacted at room temperature for 5 minutes to obtain a brown precursor solution with blue fluorescence but unstable;
[0038] (2) react the precursor solution with mercapto polyethylene glycol at room temperature in the dark for 3 hours to obtain a light yellow gold nanocluster solution that emits purple fluorescence;
[0039] (3) Dialyzing the gold nanocluster solution with a dialysis bag with a molecular weight of 2000 to obtain a pure orange-yellow fluorescent colorless gold nanocluster solution.
[0040] Wherein, the reducing agent is tyrosine, and the mercaptopolyethylene glycol is HS-C with a substance ratio of 4:1 11 -EG 6 and (11-mercaptoundecyl)hexa(ethylene glycol)methyleneoxy carboxyl (HS-C 11 -EG 6 -OCH 2 -COOH). The molar ratio of chloroauric acid, reducing agent and mercapto polyethylene glycol is 1:10:...
Embodiment 3
[0044] The preparation method of water-soluble gold nanocluster comprises the following steps:
[0045] (1) Chlorauric acid and reducing agent were reacted in the dark for 5 hours at room temperature to obtain a yellow gold nanocluster precursor solution with blue-green fluorescence but unstable;
[0046] (2) The gold nanocluster precursor reacted with mercaptopolyethylene glycol at room temperature in the dark for 12 hours to obtain a light yellow gold nanocluster solution with orange-yellow fluorescence
[0047] (3) Dialyzing the gold nanocluster solution with a dialysis bag with a molecular weight of 2000 to obtain a pure orange-yellow fluorescent colorless gold nanocluster solution.
[0048] Wherein, the reducing agent is histidine, and the mercaptopolyethylene glycol is HS-C with a substance ratio of 4:1 11 -EG 6 and (11-mercaptoundecyl)hexa(ethylene glycol)amino (HS-C 11 -EG 6 -NH 2 ). The molar ratio of chloroauric acid, reducing agent and mercapto polyethylene gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com