Palladium-doped lanthanum ferrite powder preparation method
A technology of lanthanum ferrite and powder is applied in the field of preparation of palladium-doped lanthanum ferrite powder, which can solve the problems of high price, toxicity, and no application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
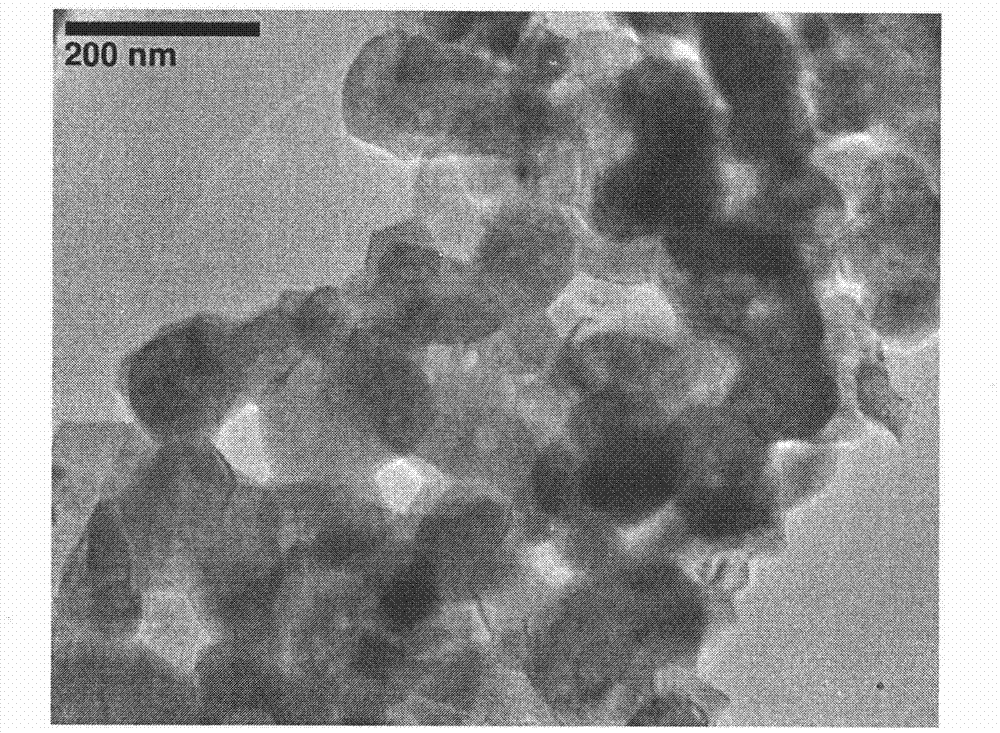
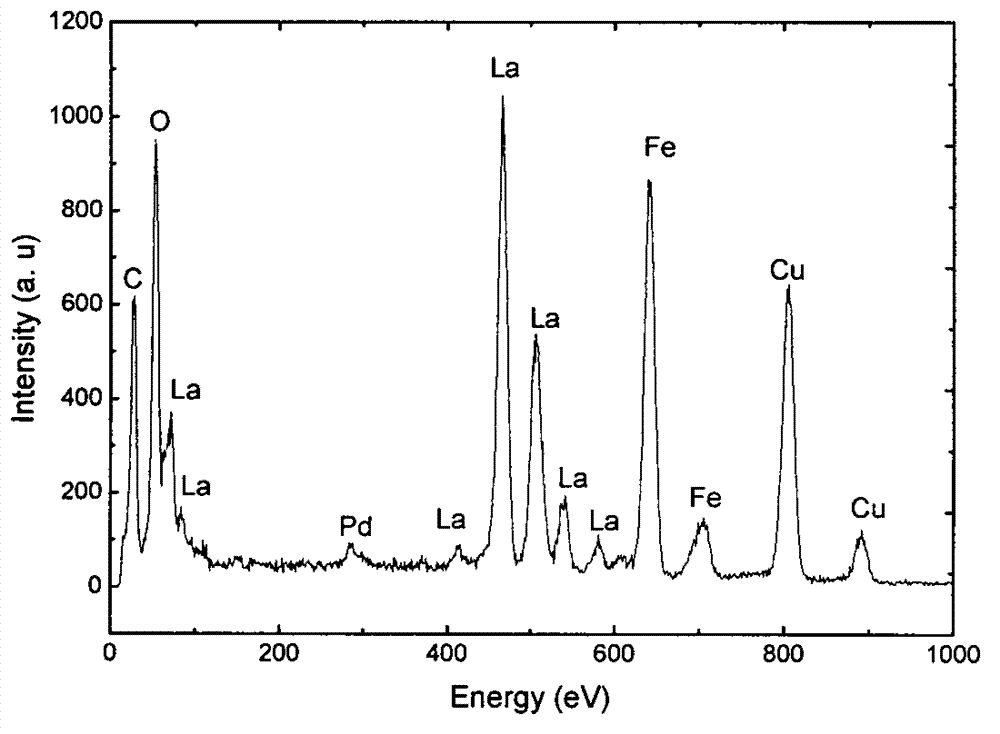
Image
Examples
Embodiment 1
[0024] Raw materials: lanthanum nitrate, iron nitrate, palladium nitrate, citric acid, all of analytical grade.
[0025] proportionally Proportioning, where x=0.05, weigh lanthanum nitrate, ferric nitrate and palladium nitrate. Dissolve lanthanum nitrate, iron nitrate and palladium nitrate in deionized water respectively, mix the three solutions, and add citric acid therein, the amount of citric acid is 1.3 times of the total molar weight of lanthanum nitrate, iron nitrate and palladium nitrate. Boiling water is refluxed to mix the solution evenly to obtain a transparent sol. After the sol evaporates part of the water in a 70°C water bath, a wet gel with a high viscosity is obtained. The wet gel is placed in a vacuum drying oven and depressurized at 60°C for 4 hours. Dried to obtain the precursor xerogel powder, and then calcined the precursor xerogel powder at 800° C. for 1 hour to obtain palladium-doped lanthanum ferrite powder.
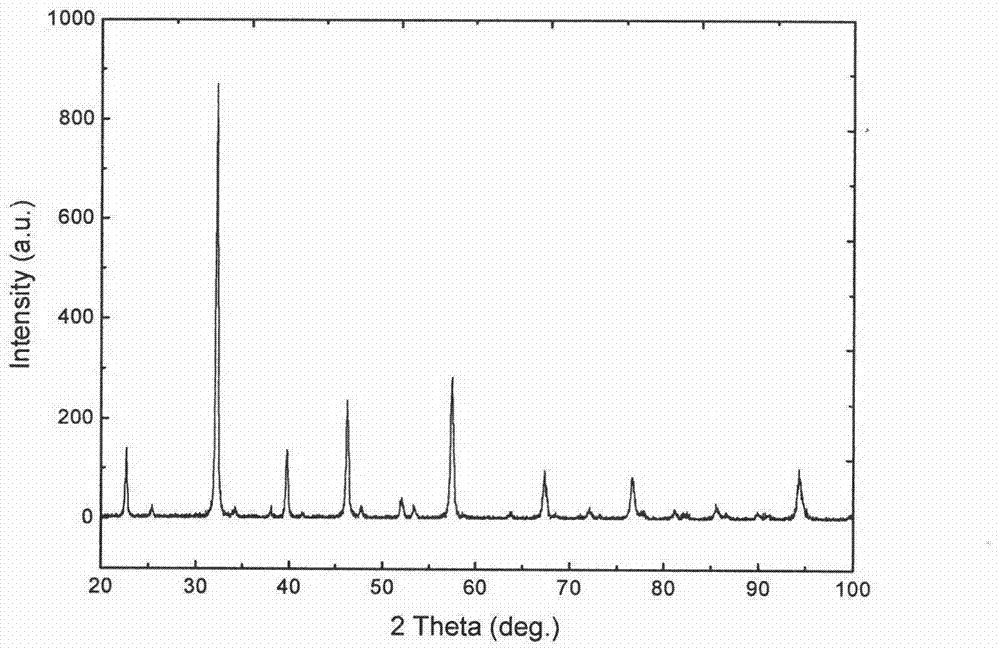
[0026] figure 1 It is the X-ray diffract...
Embodiment 2
[0028] Raw materials: Lanthanum nitrate, ferric nitrate, palladium nitrate, citric acid and polyethylene glycol 2000 are all analytically pure.
[0029] proportionally Proportioning, where x=0.1, weigh lanthanum nitrate, ferric nitrate and palladium nitrate. Dissolve lanthanum nitrate, iron nitrate and palladium nitrate with deionized water respectively, after mixing the three solutions, add citric acid and polyethylene glycol 2000 to it, the amount of citric acid is the total molar weight of lanthanum nitrate, iron nitrate and palladium nitrate The amount of polyethylene glycol 2000 is 0.05 times of the weight of citric acid, and the solution is mixed evenly by ultrasonic oscillation to obtain a transparent sol. The sol is evaporated in a 60°C water bath to obtain a wet gel with high viscosity. , the wet gel was placed in a vacuum drying oven and dried under reduced pressure at 70°C for 3 hours to obtain a precursor xerogel powder, which was then calcined at 700°C for 2 hou...
Embodiment 3
[0032] Raw materials: Lanthanum nitrate, ferric nitrate, palladium nitrate, citric acid and polyethylene glycol 20000 are all analytically pure.
[0033] proportionally Proportioning, where x=0.08, weigh lanthanum nitrate, ferric nitrate and palladium nitrate. Dissolve lanthanum nitrate, ferric nitrate and palladium nitrate with deionized water respectively, after mixing the three solutions, add citric acid and polyethylene glycol 20000 to it, the amount of citric acid is the total molar weight of lanthanum nitrate, ferric nitrate and palladium nitrate The amount of polyethylene glycol 20000 is 0.3 times of the weight of citric acid, and heated to reflux at 80°C to mix the solution evenly to obtain a transparent sol. The sol has a higher viscosity after evaporating part of the water in a water bath at 80°C. Wet gel, the wet gel is placed in a vacuum drying oven and dried under reduced pressure at 50°C for 6 hours to obtain the precursor dry gel powder, which is then roasted ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com