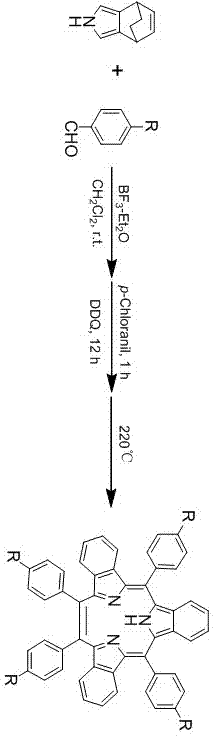
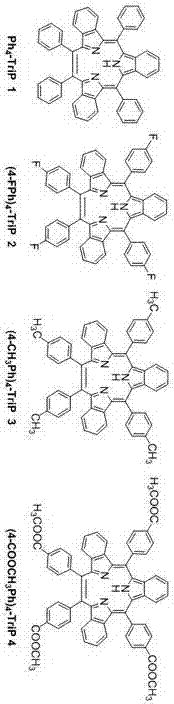
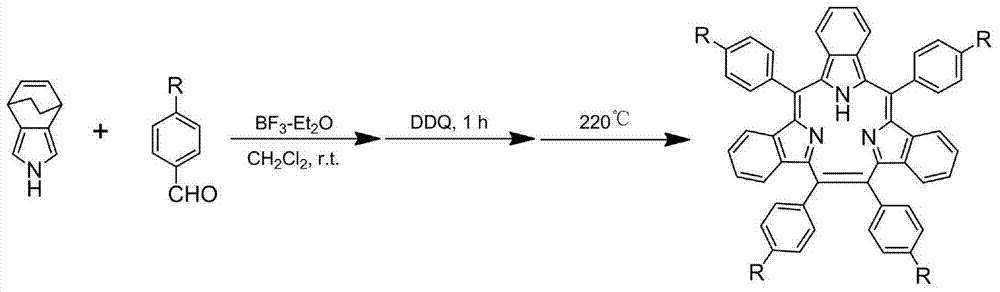
Synthesis method for triphyrin compound with no center coordination
A center-uncoordinated, ternary porphyrin technology, applied in the direction of organic chemistry, can solve problems such as constraints, and achieve the effects of improving synthesis yield, prolonging oxidation time, and simplifying the treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1, the synthesis and purification of tetraphenyl center uncoordinated ternary porphyrin
[0030] Step 1. At room temperature, add 1mmol of pyrrole to a 250ml single-necked flask, and then add 50ml of dry dichloromethane, 1mmol of benzaldehyde and 1.2mmol of boron trifluoride ether solution under the condition of aluminum foil to protect from light and nitrogen atmosphere. Stir at room temperature for 12 hours to obtain solution 1;
[0031] Step 2, adding 0.5 mmol of chlorobenzoquinone to solution 1, and oxidizing it at room temperature for 2 hours to obtain solution 2;
[0032] Step 3, add 1 mmol DDQ to solution 2, and oxidize for 10 hours at room temperature. Get solution three;
[0033] Step 4, add saturated sodium bicarbonate solution to solution 3 to quench the reaction, then wash the organic phase with distilled water and saturated brine respectively, and finally filter after drying with anhydrous sodium sulfate, and collect the filtrate;
[0034] Step 5...
Embodiment 2
[0037] Example 2, the synthesis and purification of four (4-fluorophenyl) center uncoordinated ternary porphyrins
[0038] Step 1. At room temperature, add 1mmol of pyrrole to a 250ml single-necked flask, and then add 50ml of dry dichloromethane, 1mmol of p-fluorobenzaldehyde and 1.2mmol of boron trifluoride ether solution . Stir at room temperature for 12 hours to obtain solution 1;
[0039] Step 2: Add 0.5 mmol of chlorobenzoquinone to solution 1, and oxidize it for 0.5 hours at room temperature to obtain solution 2;
[0040] Step 3: add 1 mmol DDQ to solution 2, and oxidize for 13 hours at room temperature. Get solution three;
[0041] Step 4, add saturated sodium bicarbonate solution to solution 3 to quench the reaction, then wash the organic phase with distilled water and saturated brine respectively, and finally filter after drying with anhydrous sodium sulfate, and collect the filtrate;
[0042] Step 5, the filtrate is rotary evaporated to dryness to obtain a residu...
Embodiment 3
[0045] Embodiment three, the synthesis and purification of four (4-methylphenyl) center uncoordinated ternary porphyrins
[0046]Step 1. At room temperature, add 1mmol of pyrrole to a 250ml single-necked flask, and then add 50ml of dry dichloromethane, 1mmol of p-tolualdehyde and 1.2mmol of boron trifluoride ether solution. Stir at room temperature for 12 hours to obtain solution 1;
[0047] Step 2: Add 0.5 mmol of chlorobenzoquinone to solution 1, and oxidize it at room temperature for 1 hour to obtain solution 2;
[0048] Step 3: add 1 mmol DDQ to solution 2, and oxidize for 12 hours at room temperature. Get solution three;
[0049] Step 4, add saturated sodium bicarbonate solution to solution 3 to quench the reaction, then wash the organic phase with distilled water and saturated brine respectively, and finally filter after drying with anhydrous sodium sulfate, and collect the filtrate;
[0050] Step 5, the filtrate is rotary evaporated to dryness to obtain a residue, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com