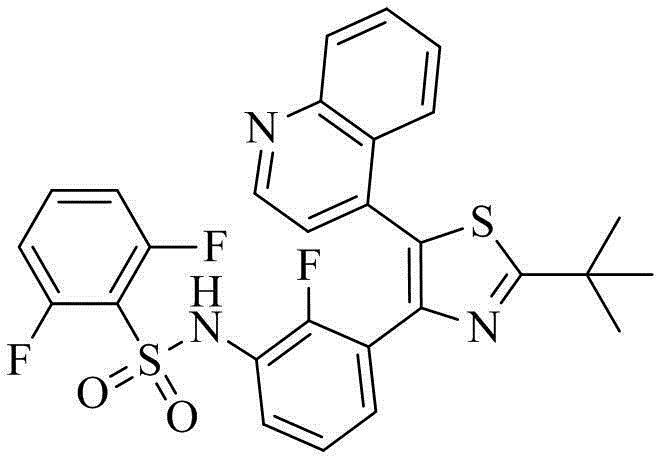
Benzenesulfonamide thiazole kinases inhibitor
An alkyl, selected technology, applied in the field of benzenesulfonamide thiazole kinase inhibitors, can solve problems such as insufficient activity and poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] (1) Preparation of SM 1
[0089] Refer to patent document WO2010 / 104899 A1, (2010).
[0090] (2) Preparation of TM 1
[0091] Dissolve SM 1 in an appropriate amount of dry THF, and replace the system with nitrogen. Place the reaction bottle in a dry ice acetone bath, cool to -78°C, slowly add DIBAL-H (3.5 equivalents) to the system dropwise, keep the system at -78°C for 6 hours, rise to room temperature and react overnight, LC-MS Monitor the progress of the reaction. After the reaction was completed, it was quenched with saturated ammonium chloride aqueous solution, extracted with ethyl acetate, the organic phases were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. The residue was separated and purified by silica gel column chromatography to obtain intermediate TM1.
[0092] (3) Preparation of TM2
[0093] The intermediate TM 1 was dissolved in an appropriate amount of dichloromethane, and excess manganese dioxide ...
Embodiment 1
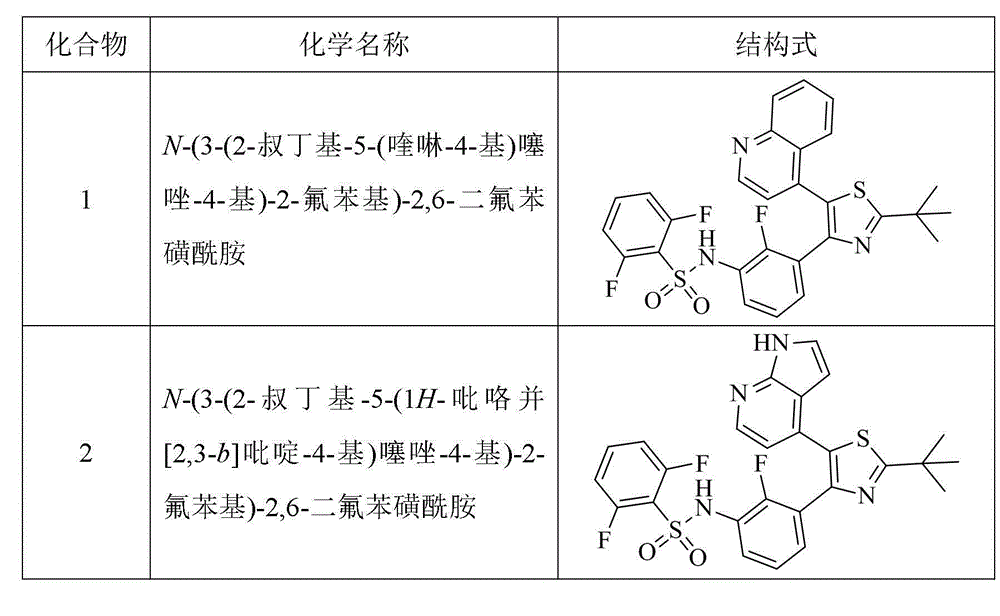
[0120] Example 1 N-(3-(2-tert-butyl-5-(quinolin-4-yl)thiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzenesulfonamide (compound 1) Preparation
[0121]
[0122] (1) Preparation of 2,2-dimethylthiopropionamide
[0123]
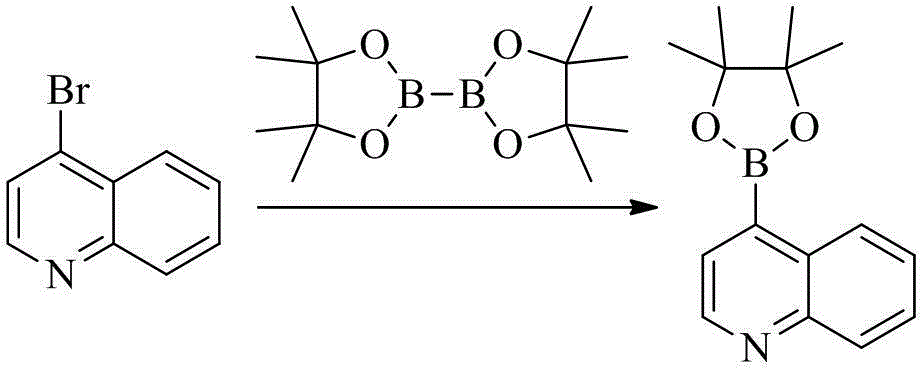
[0124] To a round bottom flask (25 mL), add 4-bromoquinoline (1 g, 4.8 mmol), double pinacol borate (1.2 g, 4.7 mmol), Pd(dppf)Cl 2 (0.1 g, 0.14 mmol), potassium acetate (0.147 g, 1.5 mmol) and 1,4-dioxane (10 mL), the system was replaced with nitrogen, heated to 90 ° C, stirred overnight, and LC-MS monitored the reaction process. After the reaction was completed, the solvent was removed by rotary evaporation, and the residue was separated and purified by silica gel column chromatography to obtain the product (0.72 g, yield 60%).
[0125] (2) Preparation of 2,6-difluoro-N-(2-fluoro-3-(hydroxymethyl)phenyl)benzenesulfonamide
[0126]
[0127] 3-(2,6-Difluorobenzenesulfonamido)-2-fluorobenzoic acid methyl ester (6.9 g, 20 mmol) was dissolved in dry THF ...
Embodiment 2
[0151] Example 2 N-(3-(2-tert-butyl-5-(1H-pyrrolo[2,3-b]pyridin-4-yl)thiazol-4-yl)-2-fluorophenyl)-2 , Preparation of 6-difluorobenzenesulfonamide (compound 2)
[0152]
[0153] (1) Preparation of 4-chloro-1-p-methylbenzenesulfonyl-1H-pyrrolo[2,3-b]pyridine
[0154]
[0155] Dissolve 4-chloro-1H-pyrrolo[2,3-b]pyridine (2 g, 13.1 mmol) in dichloromethane (60 mL), add triethylamine (3.97 g, 39.3 mmol), and then add p-toluene Sulfonyl chloride (3.75 g, 19.7 mmol) was finally added with 4-dimethylaminopyridine (220 mg, 1.8 mmol), and reacted at room temperature for 12 hours. Water was added to the system, extracted three times with ethyl acetate, and the solvent was removed by rotary evaporation to obtain a crude product (4 g, yield 99%).
[0156] (2) 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1-p-methylbenzenesulfonyl-1H-pyrrole Preparation of [2,3-b]pyridine
[0157]
[0158] Add 4-chloro-1-p-methylbenzenesulfonyl-1H-pyrrolo[2,3-b]pyridine (721 mg, 2.35 mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com