Medical biological dressing suppository for treating gynecological disease, prostate disease or anorectal disease and its application
A biological dressing and technology for gynecological diseases, applied in the field of biomedicine, can solve problems such as toxic and side effects, achieve the effect of no toxic and side effects, improve defense ability, and enhance phagocytic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
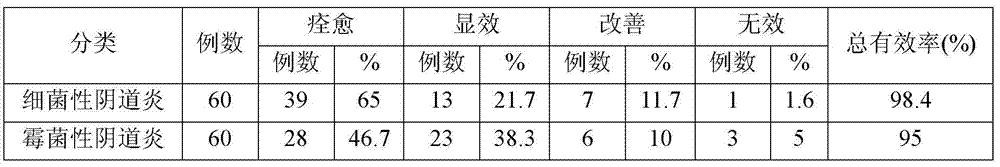
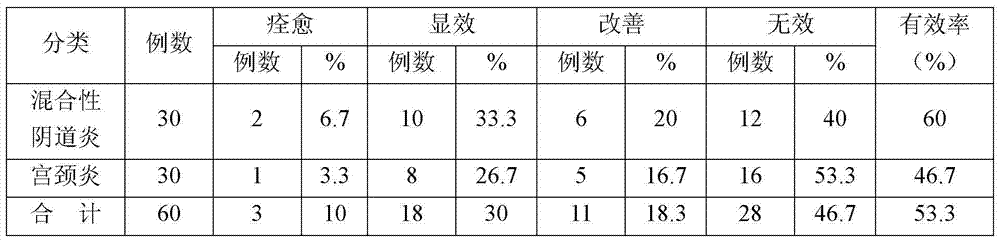
Embodiment 1
[0028] 1880 g polyethylene glycol 1500 Heat to a molten state, and then add echinacein, chitosan, and carbomer with a fineness of 100 mesh or more and 500 mesh or less 940 Stir 40 grams each to form a suspension, inject the suspension into a suppository model, and cool to obtain about 1000 suppositories of 2 grams each, which are used as the medical biological dressing suppository of Example 1.
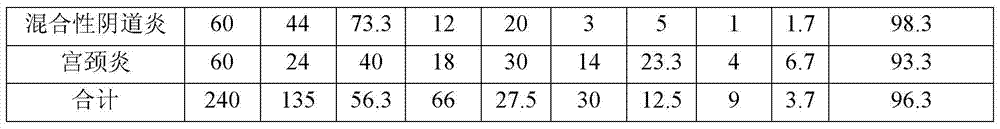
Embodiment 2
[0030] 1870 g polyethylene glycol 1500 Heat to a molten state, and then add 10 grams of echinacea, 20 grams of chitosan, and 100 grams of carbomer powder. After being evenly stirred, it was injected into a suppository model, and cooled to obtain about 1000 suppositories with 2 grams each, which were used as the medical biological dressing suppository of Example 2.
Embodiment 3
[0032] 1850 g polyethylene glycol 1500 Heat to a molten state, and then add 1 g of echinacea, 50 g of chitosan, and 100 g of carbomer powder. After being evenly stirred, it was injected into the suppository model, and cooled to obtain about 1000 suppositories of 2 grams each, which were used as the medical biological dressing suppository of Example 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com