In-vitro stress culture device for blood vessel, culture system and culture method
A culture device and culture system technology, applied in the field of in vitro stress culture devices for blood vessels, can solve the problems of poor coaxiality, inconvenient installation, liquid leakage, etc., achieve good sealing effect, prevent external leakage, and improve the effect of activity and
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
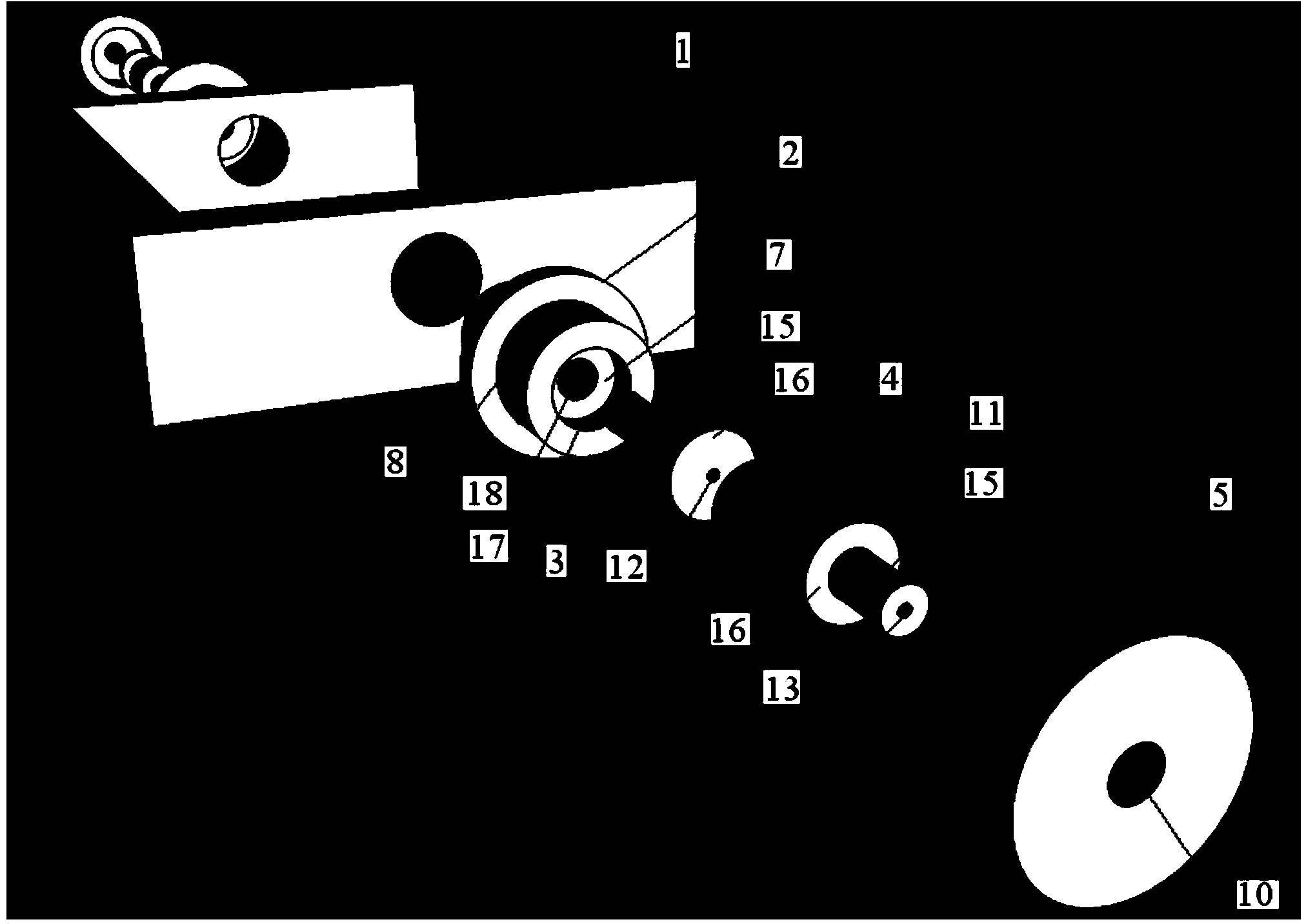
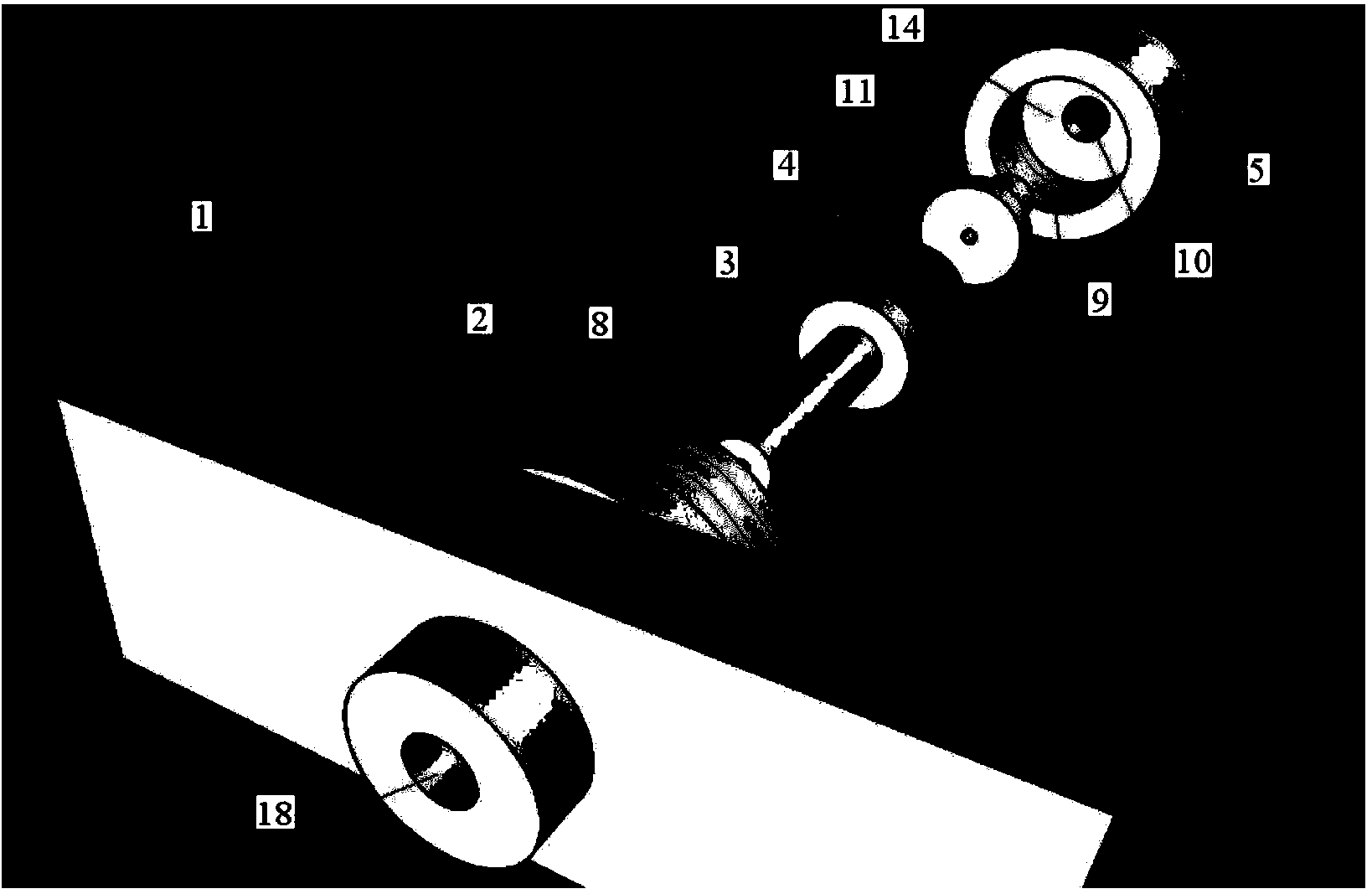
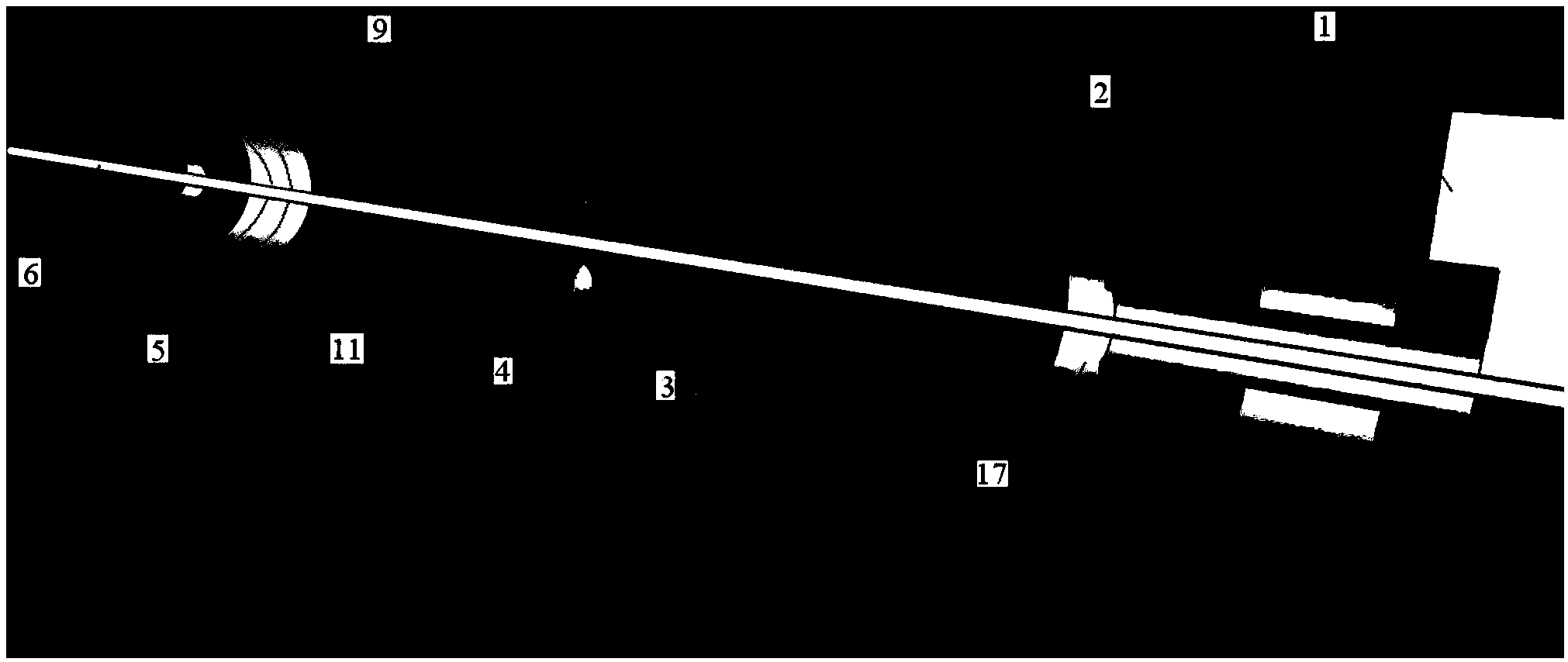
[0037] During arterial vessel culture, if the coaxiality of the blood vessel and the vascular connecting tubes at both ends is poor, the streamline form of the culture fluid (perfusate) in the culture vessel will change, which will cause certain damage to the culture vessel and change the blood vessel culture experiment. the result of. The coaxiality requirements of the blood vessel and the vascular connection tubes at both ends are related to the inner diameter of the cultured blood vessel, and the smaller the inner diameter, the higher the requirement. The inner diameter of rat arteries (≦1mm) is more than 8-10 times smaller than the inner diameters of the arteries of the existing cultured animals, so very high coaxiality is required. Moreover, most of the current fixing methods for vascular connecting tubes and culture tanks are rubber tubes with a certain wall thickness on the modified connecting tubes as the fixing and sealing materials for the culture tanks and modified ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com