Submucosal cushioning agent
A technology of swelling agent and mucous membrane, which is applied in the field of polysaccharides with pseudoplastic fluidity as its material, and can solve problems such as high viscosity and difficulty in injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The injection pressure was measured for the following injections. The method for measuring the injection pressure is as described in the "Method for Measuring the Injection Pressure of Protruding Agent" on page 6 above. Among them, the injection needles for endoscopes were measured using the following two types. It was also confirmed that it is difficult to inject the injection by hand in practice.
[0076] Endoscope injection needle 1: A puncture needle for an endoscope with a needle diameter of 23G and an effective length of the needle tube of 1600 mm (Top endoscope puncture needle for upper gastrointestinal high-flow type, manufactured by Top)
[0077] Endoscope injection needle 2: Endoscope puncture needle with a needle diameter of 23G and an effective length of the needle tube of 1650 mm (disposable injection needle DNM NM-400L-0423, manufactured by OLYMPUS)
[0078] (Preparation example of injection)
[0079] Xanthan gum (KELTROL, CGT, manufactured by CP Kelco) (hereina...
Embodiment 2
[0089] Xanthan gum performance
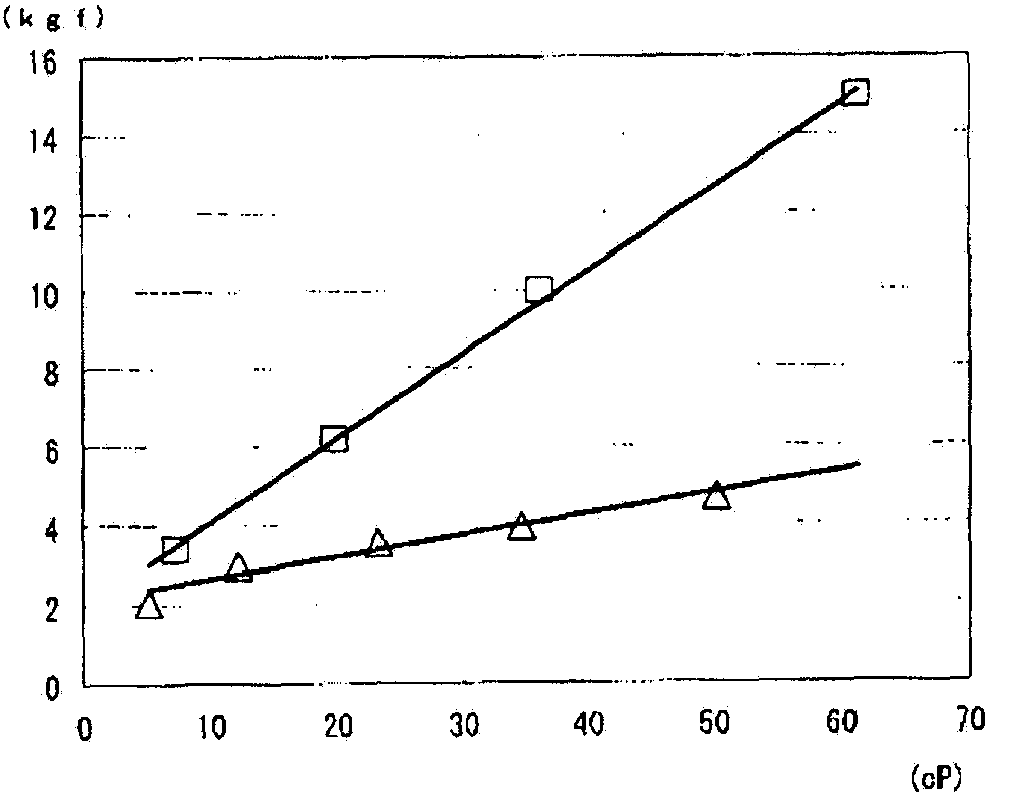
[0090] Xanthan gum (KELTROL, CGT, manufactured by CP Kelco) (hereinafter referred to as "XG") was dissolved in water for injection to prepare a 1w / v% aqueous solution. The 1 w / v% XG aqueous solution was diluted with water for injection to prepare 0.2 to 1 w / v% (concentration described in Table 3) aqueous solution. Each aqueous solution was enclosed in a vial, and the liquid after autoclaving at 121°C for 20 minutes was used as each injection. In addition, 1w / v% sodium hyaluronate (hereinafter, sodium hyaluronate abbreviated as "HA") injection (Artz intra-articular injection 25mg, manufactured by Seikagaku Kogyo) was diluted with 0.9% sodium chloride aqueous solution to prepare 0.2~ A solution of 1 w / v% (concentration described in Table 3) was used as each comparative solution. The viscosity (cP) and injection pressure (kgf) were measured for each injection and each comparative liquid. The injection pressure (kgf) was measured according to the m...
Embodiment 3
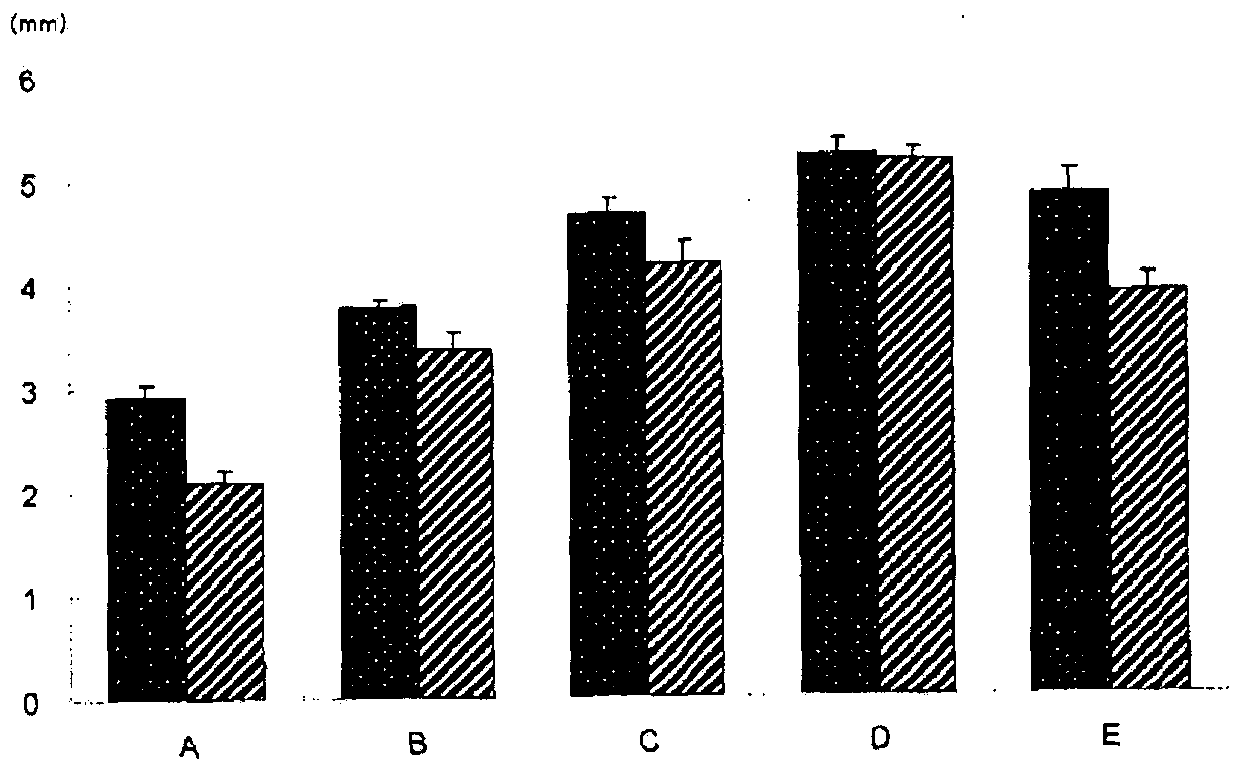
[0096] Comparison experiment of mucosal eminence
[0097] Xanthan gum (KELTROL, CGT, CP Kelco) (hereinafter referred to as "XG") was dissolved in phosphate buffered saline (pH 7.4, osmotic pressure ratio of 1) (hereinafter referred to as "PBS") to prepare 1w / v% XG in PBS. Dilute the 1w / v% XG PBS solution with PBS to prepare 0.5w / v% XG and 0.25w / v% XG PBS solutions. The XG PBS solution of each concentration was sealed in a vial, and the liquid after autoclaving at 121°C for 20 minutes was used as each injection. The pH, osmotic pressure ratio, viscosity (cP) and injection pressure (kgf) were measured for each injection and 0.4w / v% solution (MucoUp, manufactured by Seikagaku Kogyo). The injection pressure (kgf) was measured according to the method described in "Method for Measuring the Injection Pressure of Heave Agent" on page 6 above. Among them, as an injection needle for an endoscope, a puncture needle for an endoscope (disposable injection needle DNM NM-400L-0423, manufactu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com