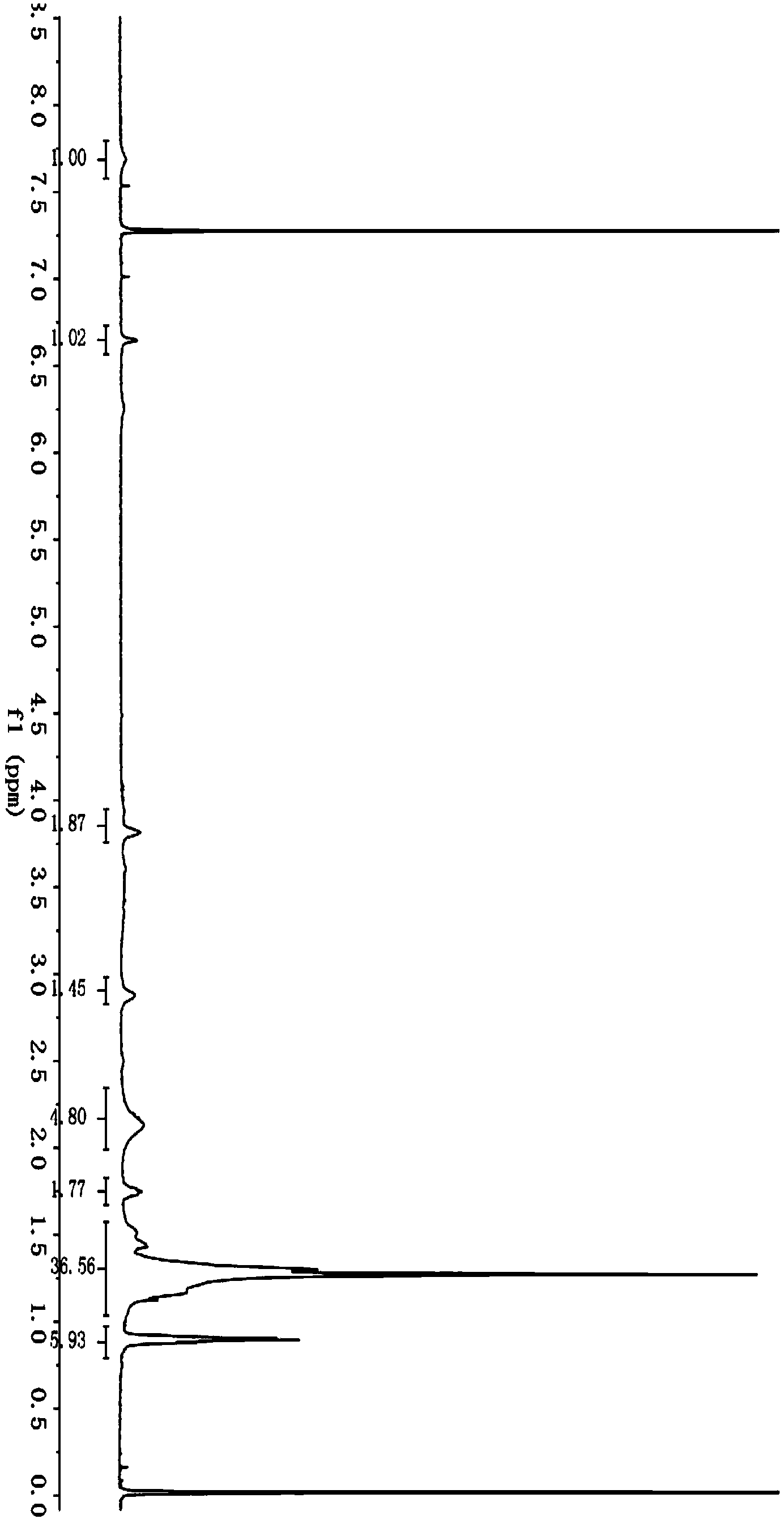Saturated anacardol ether sulfonate surfactant as well as preparation method and application thereof
A technology of cardanol ether sulfonate and surfactant, applied in the field of saturated cardanol ether sulfonate surfactant and its preparation, capable of solving unmentioned problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
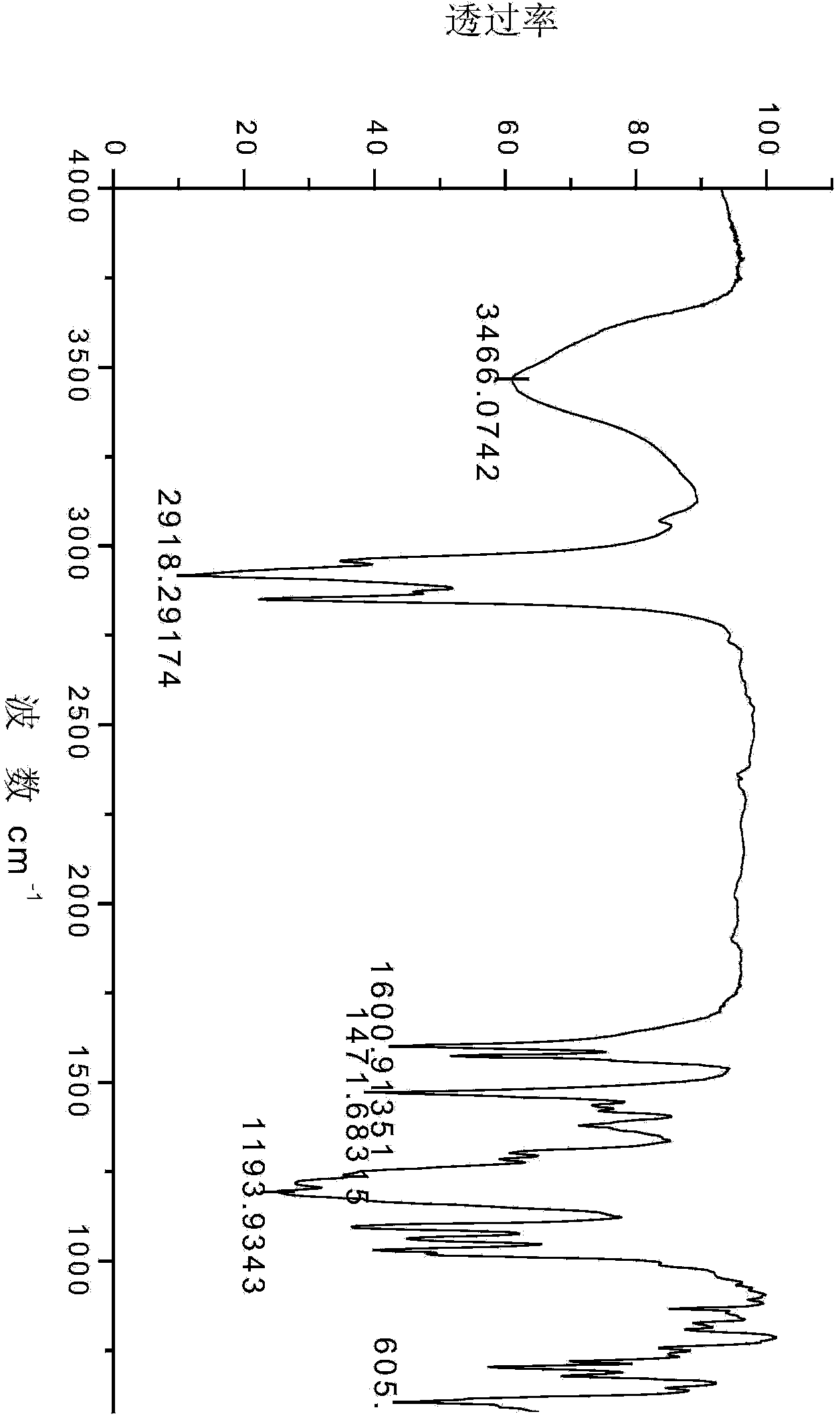
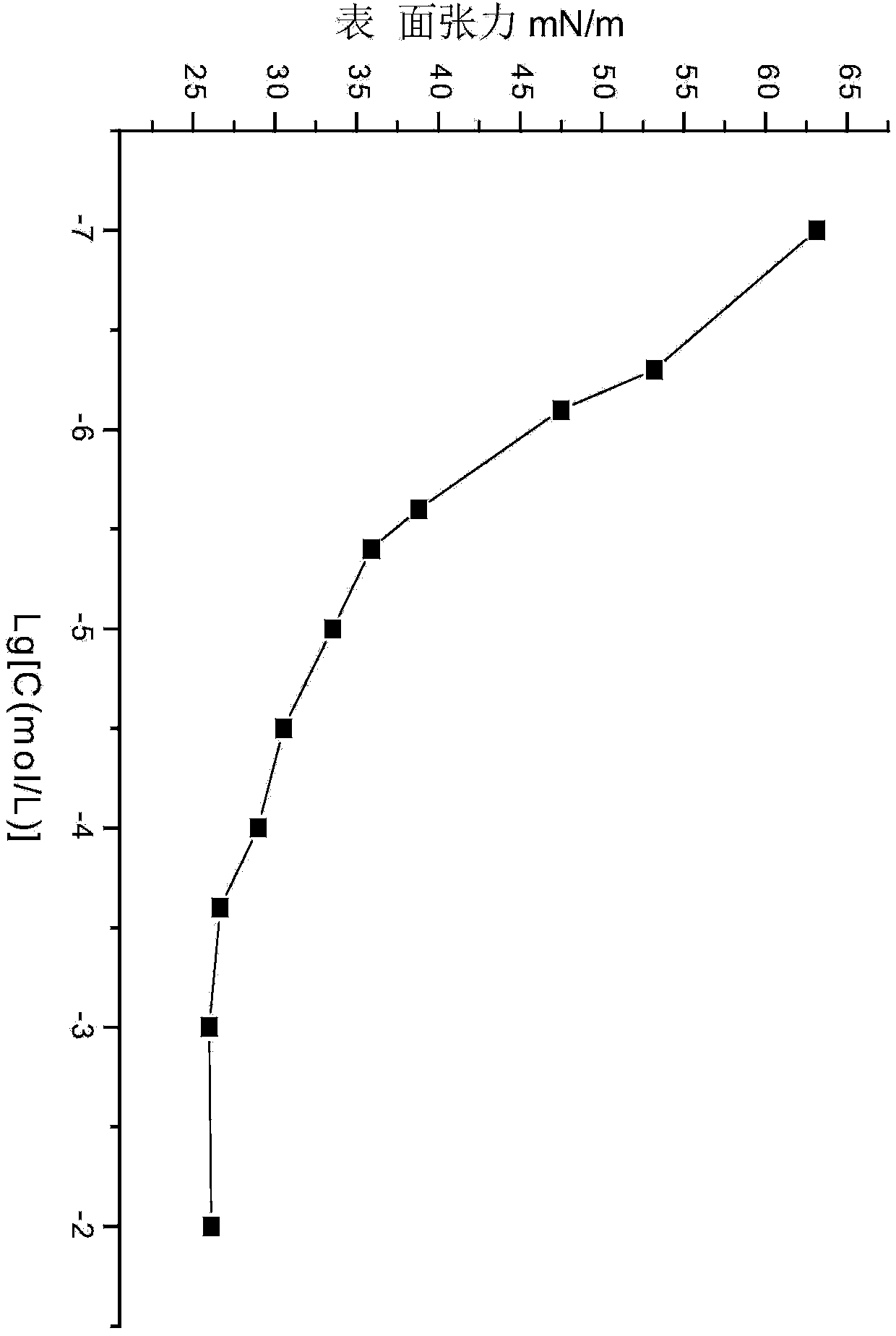
[0042] The preparation and application of embodiment 1 saturated cardanol butyl ether sulfonic acid sodium salt
[0043] (1). Synthesis of saturated cardanol butyl ether
[0044]At room temperature, add 0.025mol (7.61g) of saturated cardanol, 0.030mol (4.11g) of bromobutane and 0.2g of phase to a 50mL three-necked flask equipped with electromagnetic stirring, a thermometer, and a constant pressure dropping funnel. Transfer catalyst tetrabutylammonium bromide, heat at 80°C, add dropwise 6.25g of sodium hydroxide aqueous solution with a concentration of 40wt%, after the dropwise addition of the sodium hydroxide aqueous solution, carry out a reflux reaction for 6 hours, after the reflux reaction is completed, add 60mL of water , extracted 3 times with 80 mL of petroleum ether, combined the petroleum ether extracts, and carried out back-extraction with 50 mL of water for 3 times; dry the petroleum ether phase after back-extraction with water with anhydrous sodium sulfate, filter, ...
Embodiment 2
[0048] The preparation of embodiment 2 saturated cardanol decyl ether sulfonic acid sodium salt and its application
[0049] (1). Synthesis of saturated cardanol decyl ether
[0050] At room temperature, 0.025mol (7.61g) of saturated cardanol, 0.036mol (7.96g) of bromodecane and 0.15g phase transfer were added to a 50mL three-necked flask equipped with electromagnetic stirring, a thermometer and a constant pressure dropping funnel. The catalyst, tetrabutylammonium bromide, was heated at 85°C, and 6.5 g of sodium hydroxide aqueous solution with a concentration of 40 wt% was added dropwise. After the sodium hydroxide aqueous solution was added dropwise, a reflux reaction was carried out for 7 hours. After the reflux reaction was completed, 60 mL of water was added. Extract 3 times with 80 mL of petroleum ether, combine the petroleum ether extracts, and carry out back-extraction with 50 mL of water for 3 times; dry the petroleum ether phase after back-extraction with water with a...
Embodiment 3
[0054] The preparation of embodiment 3 saturated cardanol cetyl ether sulfonic acid sodium salt
[0055] (1). Synthesis of saturated cardanol cetyl ether
[0056] At room temperature, add 0.025mol (7.61g) of saturated cardanol, 0.032mol (9.76g) of bromohexadecane and 0.2g to a 50mL three-necked flask equipped with electromagnetic stirring, a thermometer and a constant pressure dropping funnel. The phase transfer catalyst tetrabutylammonium chloride was heated at 80°C, and 6.0 g of sodium hydroxide aqueous solution with a concentration of 40 wt % was added dropwise. After the sodium hydroxide aqueous solution was added dropwise, a reflux reaction was carried out for 7 hours. After the reflux reaction was completed, 60 mL of Water, extracted 3 times with 80 mL of petroleum ether, combined the petroleum ether extracts, and carried out back-extraction with 50 mL of water for 3 times; dry the petroleum ether phase after back-extraction with water over anhydrous sodium sulfate, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com