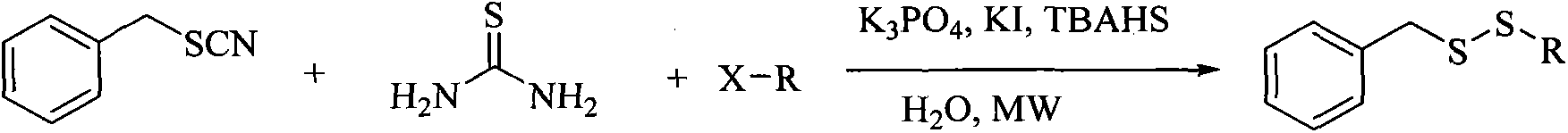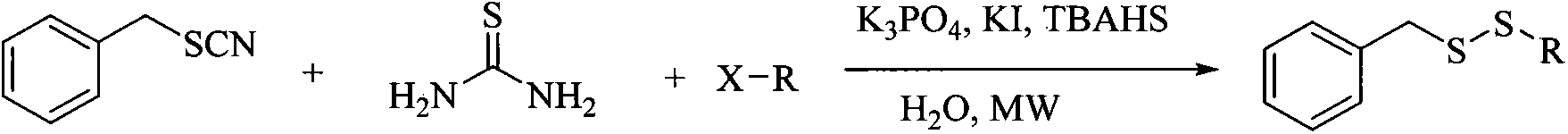Method for synthesizing benzyl alkyl disulfide
A technology for synthesizing benzyl alkyl disulfides and alkyl halides, which is applied in the field of synthesis of benzyl alkyl disulfides, can solve the problem that the asymmetric disulfide reaction has not been further explored, has a relatively irritating odor, It is not easy to scale production and other problems, to achieve the effect of saving energy consumption, low price and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of Benzyl Octyl Disulfide
[0020] Benzyl thiocyanide 149mg (1mmol), 1-bromooctane 288mg (1.5mmol), thiourea 152mg (2mmol), potassium iodide 248mg (1.5mmol), tetrabutylammonium bisulfate 509mg (1.5mmol) and potassium phosphate 207mg (1.2mmol) added to 3mL H 2 In the reaction vial of O, the reaction temperature was set at 90°C and stirred by microwave. Set the reaction time to 15min, and stop the reaction when the time is over. The resultant was extracted with DCM, and the organic phases were combined, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain a crude product. Purified by silica gel column, eluent: n-hexane, and isolated to obtain benzyl octyl disulfide.
[0021] Benzyl octyl disulfide is a colorless oil with a yield of 61%.
[0022] GC-MS: [C 15 h 24 S 2 +H] + The calculated value of is 269.1319 and the measured value is 269.0130.
[0023] 1 H NMR (400MHz, CD 3 OD) δ: 7.38-7.14 (m, 5H), 3.88 (s, 2H), 2.34 (t, J=7....
Embodiment 2
[0026] Preparation of Benzyl Heptyl Disulfide
[0027] Benzyl thiocyanide 149mg (1mmol), 1-bromoheptane 267mg (1.5mmol), thiourea 152mg (2mmol), potassium iodide 248mg (1.5mmol), tetrabutylammonium bisulfate 509mg (1.5mmol) and potassium phosphate 207mg (1.2mmol) added to 3mL H 2 In the reaction vial of O, the reaction temperature was set at 90°C and stirred by microwave. Set the reaction time to 15min, and stop the reaction when the time is over. The resultant was extracted with DCM, and the organic phases were combined, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain a crude product. Through silica gel column purification, eluent: n-hexane, benzyl heptyl disulfide was isolated.
[0028] Benzylheptyl disulfide is a colorless oily substance with a yield of 88%.
[0029] GC-MS: [C 14 H2 2 S 2 +H] + The calculated value is 255.1163, and the measured value is 254.9696.
[0030] 1 H NMR (600MHz, CD 3 OD)δ: 7.37-7.26(m, 5H), 3.91(s, 2H), 2.39-...
Embodiment 3
[0033] Preparation of Benzyl Hexyl Disulfide
[0034] Benzyl thiocyanide 149mg (1mmol), 1-bromohexane 246mg (1.5mmol), thiourea 152mg (2mmol), potassium iodide 248mg (1.5mmol), tetrabutylammonium bisulfate 509mg (1.5mmol) and potassium phosphate 207mg (1.2mmol) added to 3mL H 2 In the reaction vial of O, the reaction temperature was set at 80 °C and stirred by microwave. Set the reaction time to 15min, and stop the reaction when the time is over. The resultant was extracted with DCM, and the organic phases were combined, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain a crude product. Purify by silica gel column, eluent: n-hexane, and isolate benzyl hexyl disulfide.
[0035] Benzylhexyl disulfide is a colorless oil with a yield of 85%.
[0036]GC-MS: [C 13 h 20 S 2 +H] + The calculated value of is 241.1006 and the measured value is 241.9353.
[0037] 1 H NMR (400MHz, CD 3 OD) δ: 7.35-7.23 (m, 5H), 3.88 (s, 1H), 2.35 (t, J = 7.3Hz, 2H), 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com