Polymorphic substance of pyridinium derivative used as M3 muscarinic receptor antagonist as well as preparation method and medicine composition of polymorphic substance
A technology of pyrrolidine and crystal forms, which is applied in the field of preparation of drugs for urinary system diseases or gastrointestinal diseases, 3R-1, to prevent or treat respiratory diseases, and can solve the problem of poor stability and fluidity of solid powders and difficulties in lung preparations Uncertainty, insufficient crystallization and other problems, to achieve good performance and suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Form A of (R)-1,1-dimethyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidinium bromide (compound of formula I)
[0059] Dissolve (R)-1-methyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidine (1.0g, 3.1mmol) in 4mL butanone, under ice-cooling Methyl bromide (588mg, 6.2mmol) was added dropwise, and the temperature was slowly raised to room temperature. After reacting overnight, a solid precipitated out. The solid was filtered with suction, and the filter cake was washed with methyl ethyl ketone to obtain an off-white solid (0.98g), melting point: 224°C-227°C. 1 H NMR (DMSO, 500MHz) 67.51 (m, 2H), 7.48 (s, 1H), 7.13 (m, 2H), 7.00 (m, 2H), 5.52 (m, 1H), 3.93 (m, 1H), 3.73 (m, 2H), 3.63(m, 1H), 3.20(s, 3H), 3.03(s, 3H), 2.74(m, 1H), 2.16(m, 1H); 13 C NMR (DMSO, 125MHz) 6170.33, 146.63, 126.69, 126.24, 125.75, 125.70, 76.24, 73.85, 69.09, 63.94, 52.66, 51.91, 29.74; EI-MS m / z 338.1 [M-Br] + . The solid product was confirmed to be crystal form A of the compound of formu...
Embodiment 2
[0061] Form B of (R)-1,1-dimethyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidinium bromide (compound of formula I)
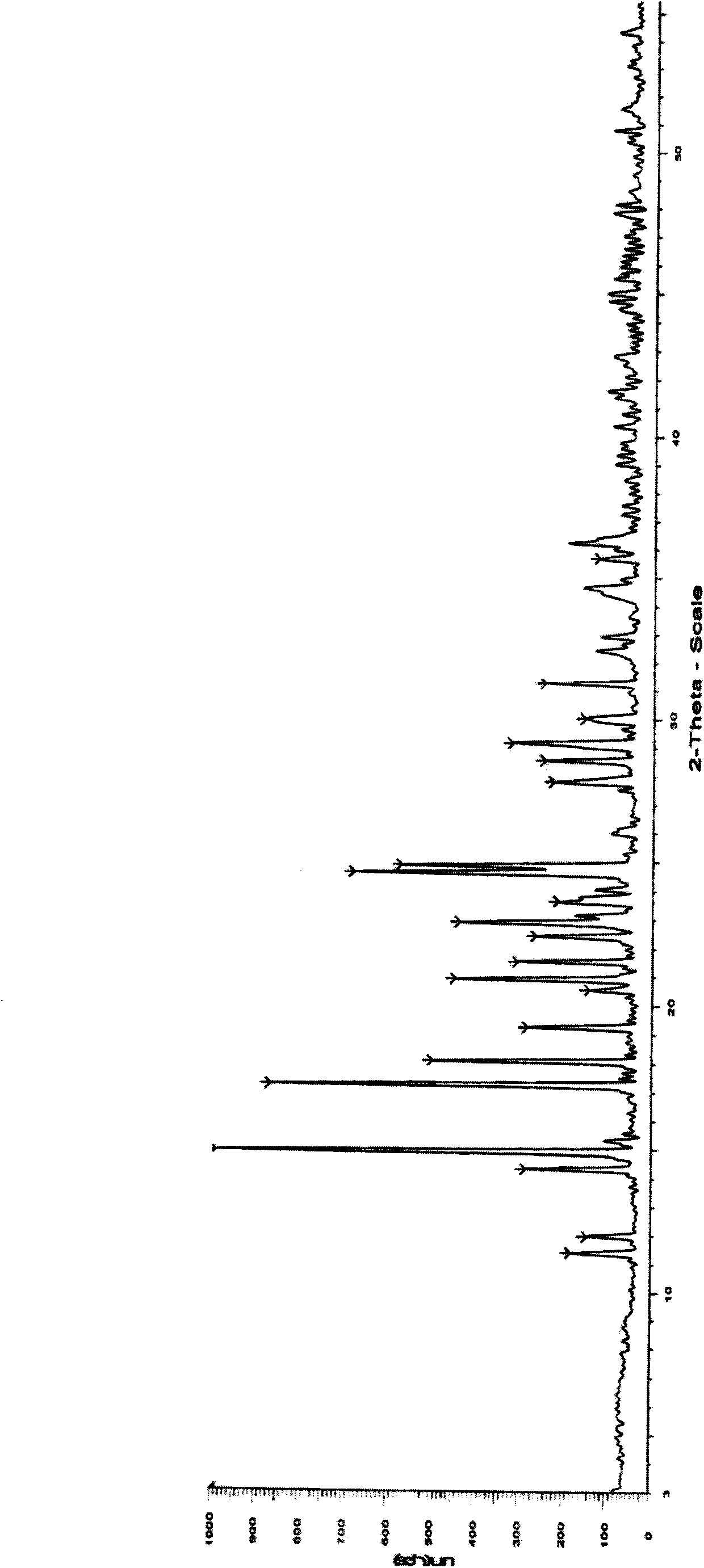
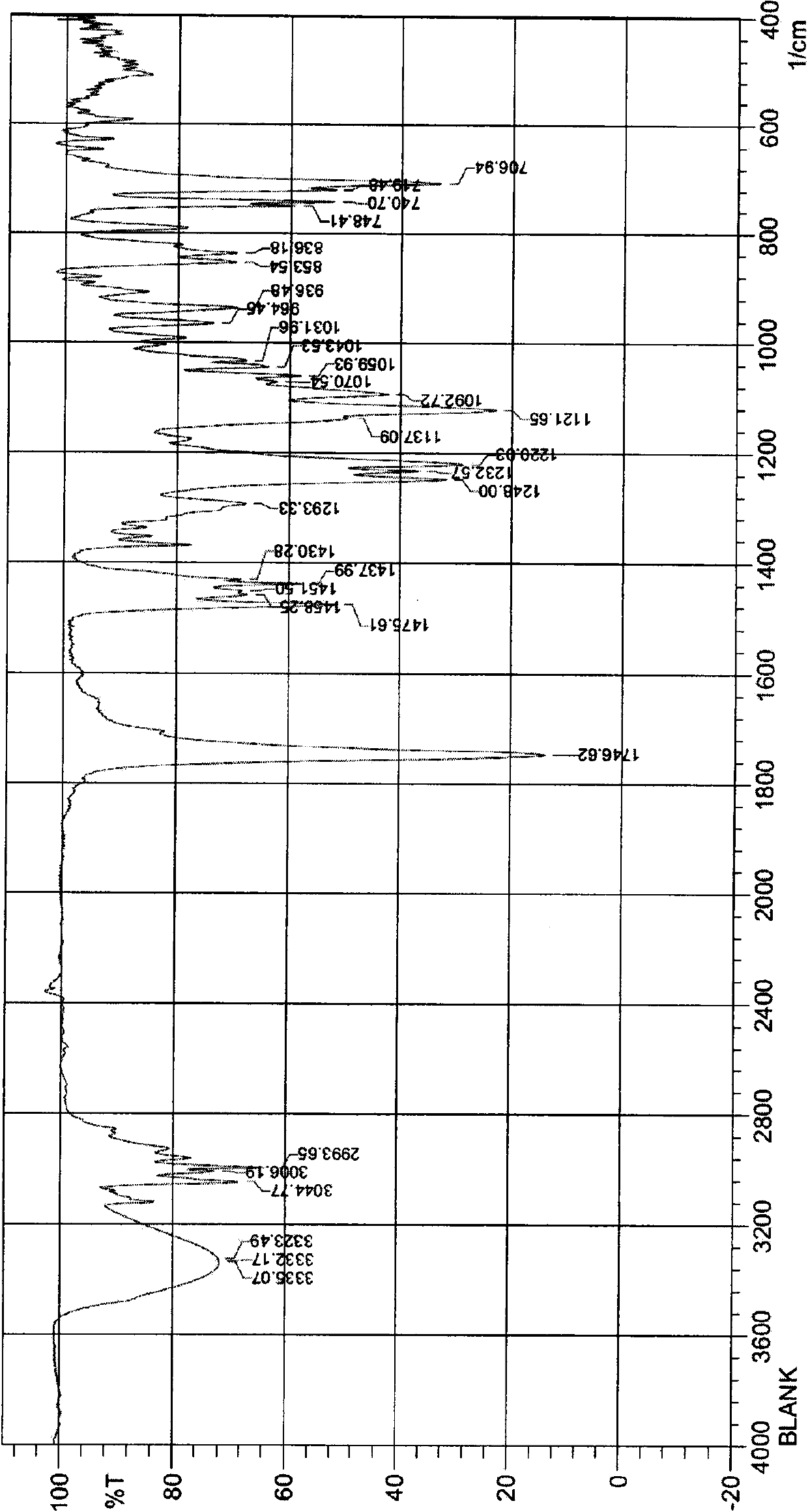
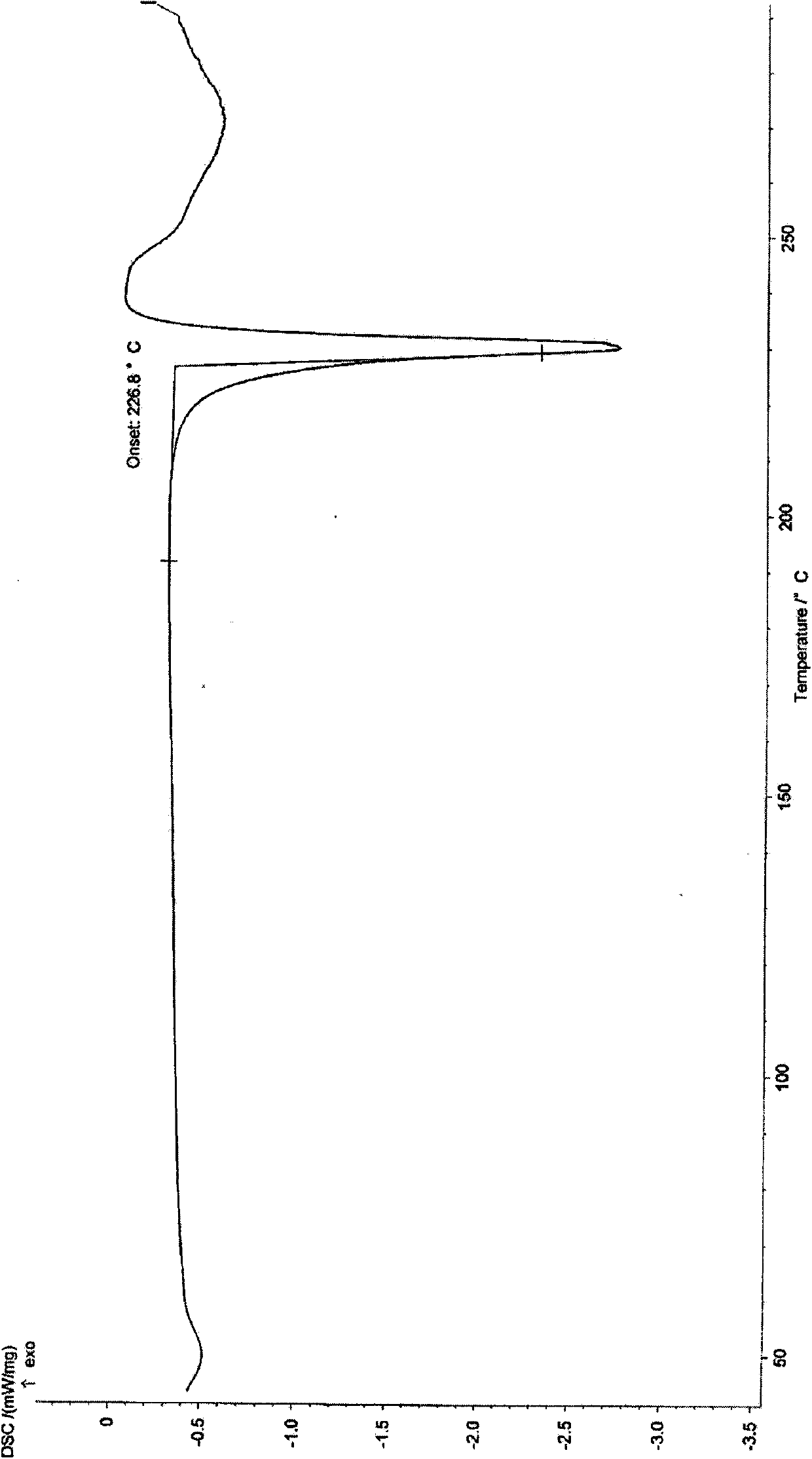
[0062] Dissolve (R)-1-methyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidine (200mg, 0.62mmol) in 0.4mL dichloromethane, ice bath Methyl bromide (118mg, 1.24mmol) was added dropwise, and the temperature was slowly raised to room temperature. After reacting overnight, a solid precipitated out. The solid was filtered with suction, and the filter cake was washed with dichloromethane to obtain an off-white solid (245mg, 94%). Melting point: 227°C ~230°C. The solid product is confirmed to be crystal form B of the compound of formula I through X-ray powder diffraction, infrared scanning, DSC scanning and TGA scanning.
Embodiment 3
[0064] Form B of (R)-1,1-dimethyl-3-(2-hydroxy-2,2-dithiophen-2-ylacetoxy)pyrrolidinium bromide (compound of formula I)
[0065] Dissolve the crystalline form A (300mg) of the compound of formula I in 4mL of a mixed solvent of ethanol and water (19:1) at a temperature of 80°C under stirring, then slowly cool to 15°C and let it stand, a white color precipitates out of the solution crystals. After filtering, the filter cake was washed with a small amount of ethanol, and the collected crystals were dried under reduced pressure at 45°C to obtain 278 mg of white crystals, melting point: 226°C-229°C. The crystal is confirmed to be crystal form B of the compound of formula I through X-ray powder diffraction, infrared scanning, DSC scanning, TGA scanning and elemental analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com