Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
A phosphorescent material, metal iridium technology, applied in luminescent materials, electro-solid devices, organic chemistry, etc., can solve the problems of limited carrier transport, low luminous efficiency, poor stability, etc., to increase solubility and luminous efficiency. High and convenient synthesis and processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
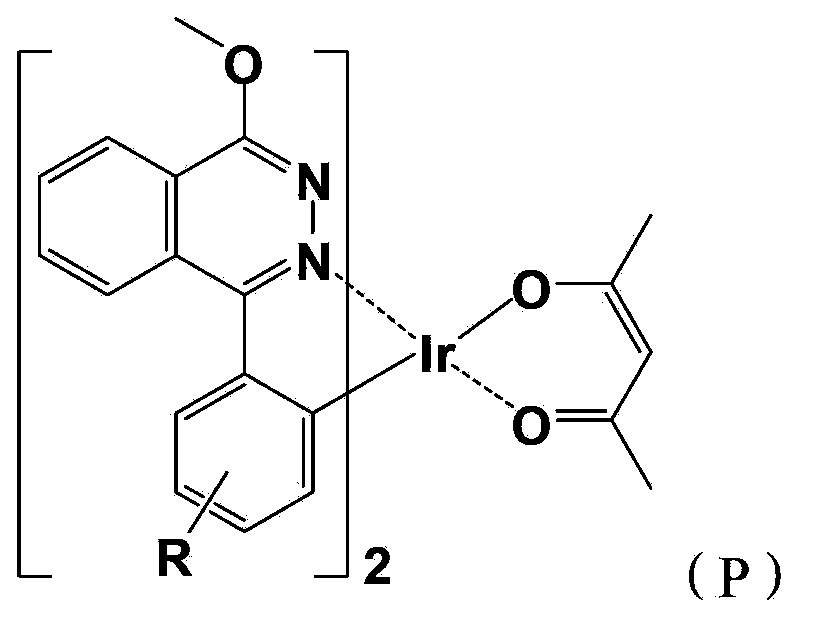
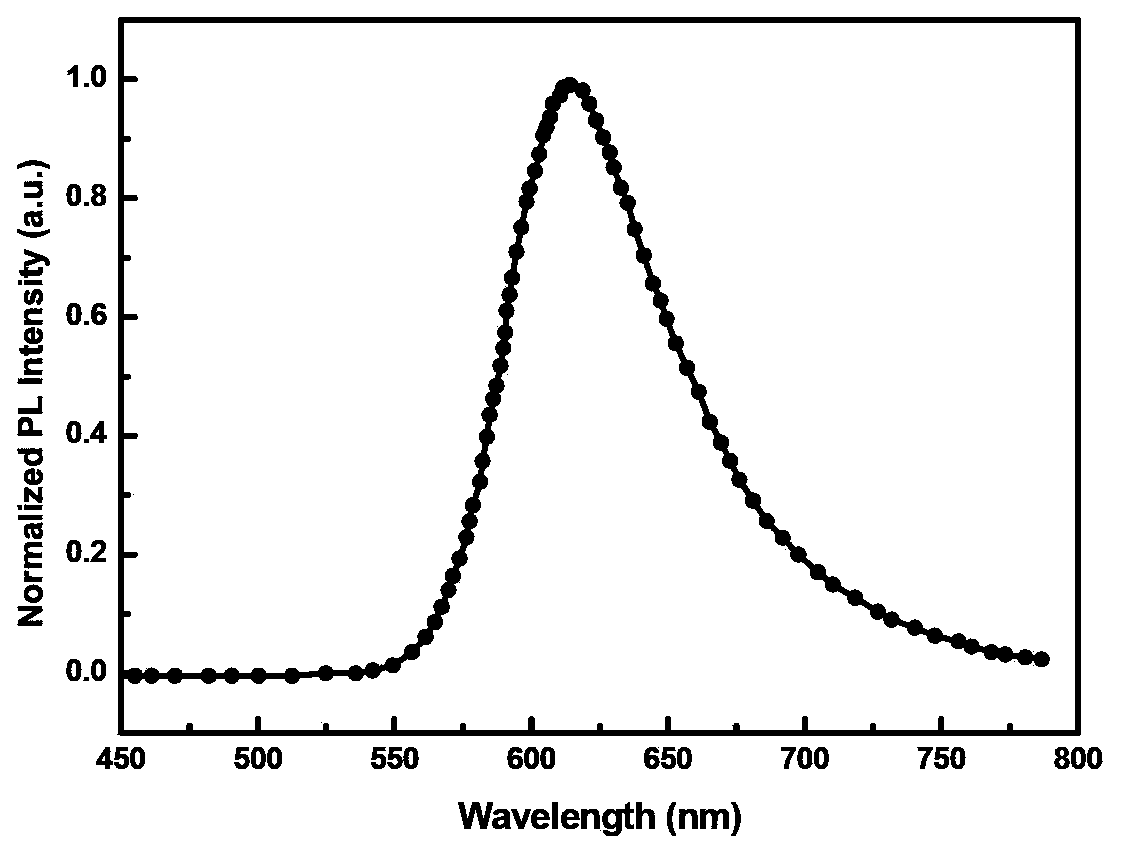
[0059] A red-light organic electrophosphorescent material metal iridium complex bis[1-methoxy-4-phenylphthalazine-N,C 2 '](acetylacetonate) iridium, with 1-methoxy-4-phenylphthalazine as ring metal ligand, as shown in the following structural formula:
[0060]
[0061] Wherein, the R group is a hydrogen atom (not shown in the structural formula), and the structural formula of the ring metal ligand is
[0062]
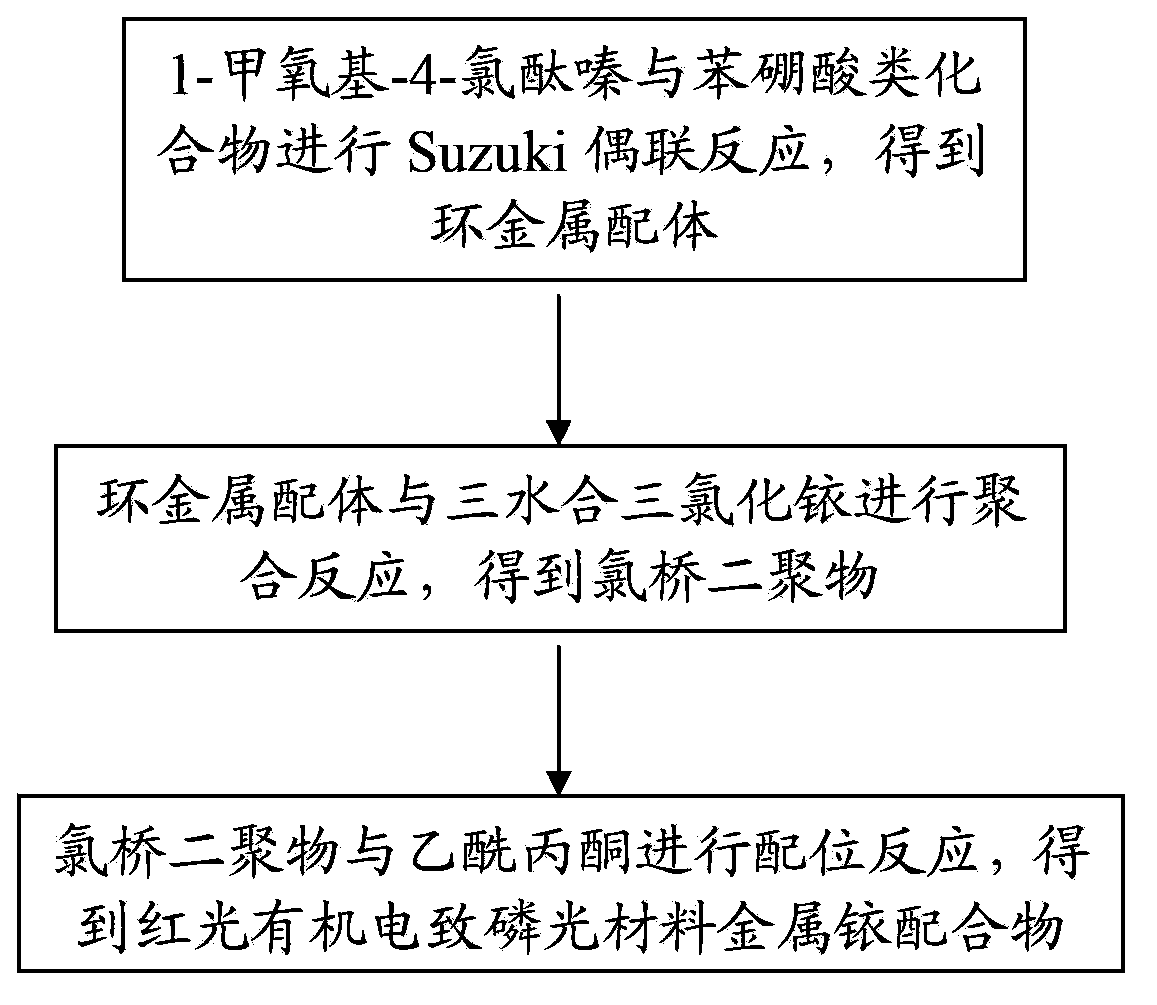
[0063] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0064] (1) Provide compound A and compound B1 represented by the following structural formula respectively:
[0065]
[0066] (2) Synthesis of cyclometal ligand 1-methoxy-4-phenylphthalazine
[0067] Under the protection of nitrogen, 0.78g (4.0mmol) 1-methoxy-4-chlorophthalazine (ie compound A), 0.59g (4.8mmol) phenylboronic acid (ie compound B1) and 0.23g (0.20mmol) four (Triphenylphosphine) pall...
Embodiment 2
[0090] A red-light organic electrophosphorescent material metal iridium complex bis[1-methoxy-4-(6'-methylphenyl)phthalazine-N,C 2 '](acetylacetonate) iridium, with 1-methoxy-4-(2'-methylphenyl)phthalazine as the ring metal ligand, as shown in the following structural formula:
[0091]
[0092] In the formula, the R group is a methyl group, and the structural formula of the ring metal ligand is
[0093]
[0094] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0095] (1) Provide compound A and compound B2 represented by the following structural formula respectively:
[0096]
[0097] (2) Synthesis of cyclometal ligand 1-methoxy-4-(2'-methylphenyl)phthalazine
[0098] Under nitrogen protection, 0.78g (4.0mmol) 1-methoxy-4-chlorophthalazine (ie compound A), 1.09g (8mmol) 2-methylphenylboronic acid (ie compound B2) and 0.23g (0.20 mmol) tetrakis (triphenylphosph...
Embodiment 3
[0120] A red-light organic electrophosphorescent material metal iridium complex bis[1-methoxy-4-(5'-methylphenyl)phthalazine-N,C 2 '](acetylacetonate) iridium, with 1-methoxy-4-(3'-methylphenyl)phthalazine as the ring metal ligand, as shown in the following structural formula:
[0121]
[0122] In the formula, the R group is a methyl group, and the structural formula of the ring metal ligand is
[0123]
[0124] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0125] (1) Provide compound A and compound B3 represented by the following structural formula respectively:
[0126]
[0127] (2) Synthesis of cyclometal ligand 1-methoxy-4-(3'-methylphenyl)phthalazine
[0128] Under nitrogen protection, 0.78g (4.0mmol) 1-methoxy-4-chlorophthalazine (ie compound A), 0.55g (4mmol) 3-methylphenylboronic acid (ie compound B3) and 0.14g (0.20 mmol) dichlorobis(triphenylphos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com