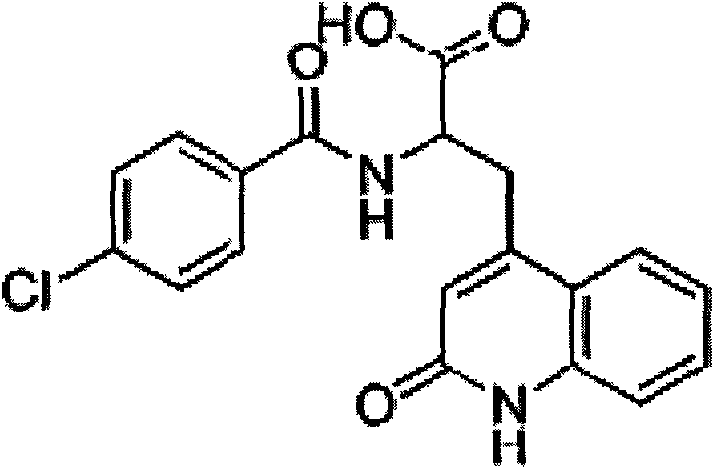
Rebamipide suspension eye drop containing water soluble high-molecular polymers and preparation method thereof
A technology of water-soluble polymer and rebamipide, which is applied in the field of medicine, can solve problems such as inability to make transparent and clear eye drops, poor water solubility of rebamipide, and discomfort for patients, and achieve a simple and easy-to-control production process , the source is easy to obtain, and the effect of reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] prescription
[0035]
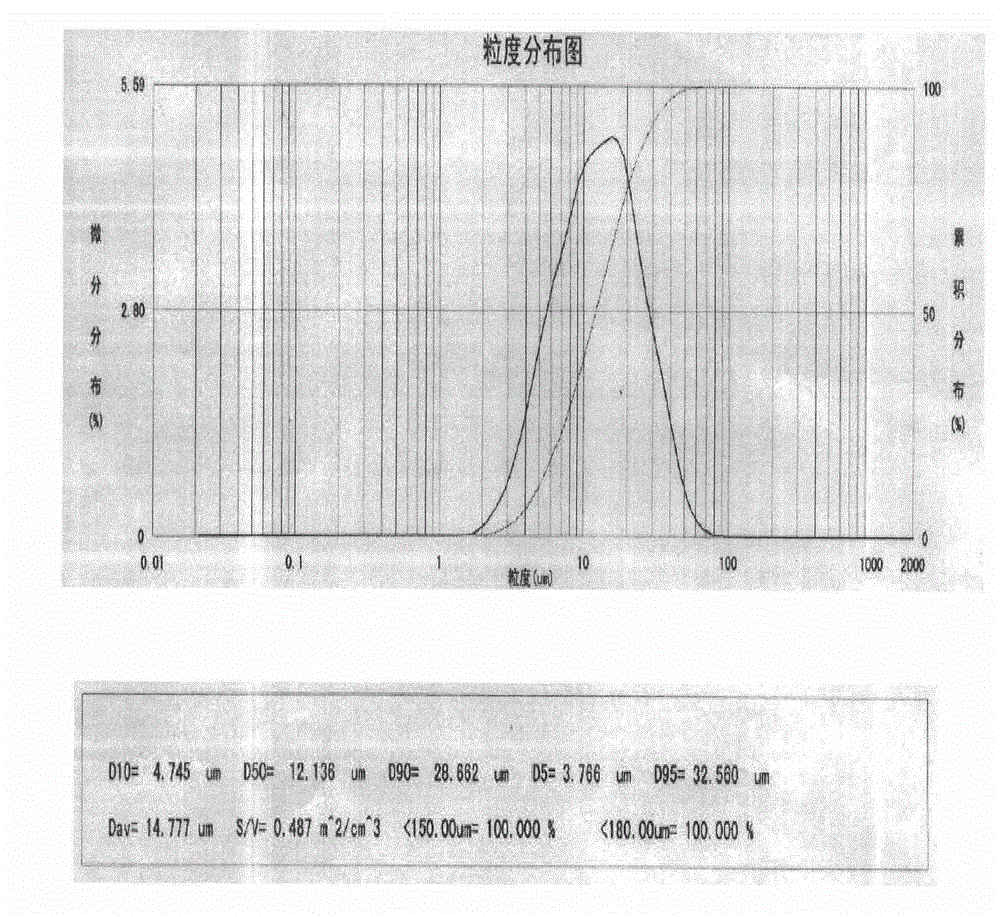
[0036] Take an appropriate amount of water for injection, heat it to 80°C to 90°C, weigh carboxymethylcellulose sodium and disperse it in it, stir to dissolve, add disodium ethylenediaminetetraacetate and sodium chloride, stir to dissolve, use sodium hydroxide / hydrochloric acid to adjust the pH value to 6.5-7.5, sterilize and filter with a 0.2 μm microporous membrane, add sterile rebamipide ultrafine pulverized particles (0.1-50 μm), stir properly, mix well, add water for injection to the full amount, Shake well and dispense it into eye drop bottles under sterile conditions.
Embodiment 2
[0038] prescription
[0039]
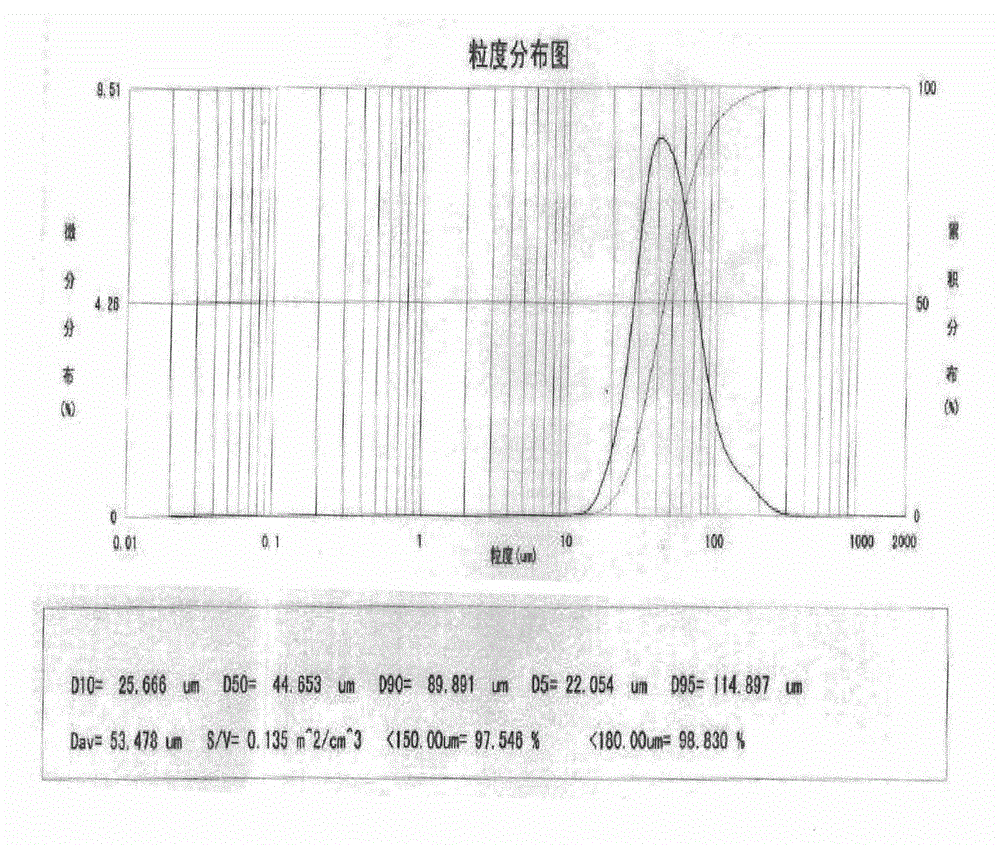
[0040] Take an appropriate amount of water for injection, heat it to 80°C to 90°C, weigh carboxymethylcellulose sodium and disperse it in it, stir to dissolve, add disodium ethylenediaminetetraacetate and sodium chloride, stir to dissolve, use sodium hydroxide / hydrochloric acid to adjust the pH value to 6.5-7.5, sterilize and filter with a 0.2 μm microporous membrane, add sterile rebamipide ultrafine pulverized particles (0.1-50 μm), stir properly, mix well, add water for injection to the full amount, Shake well and dispense it into eye drop bottles under sterile conditions.
Embodiment 3
[0042] prescription
[0043]
[0044]
[0045]Prepare with reference to the method of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com