Preparation method of insulin
A technology of insulin and trypsin, applied in the direction of fermentation, etc., can solve the problems of impracticality in large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
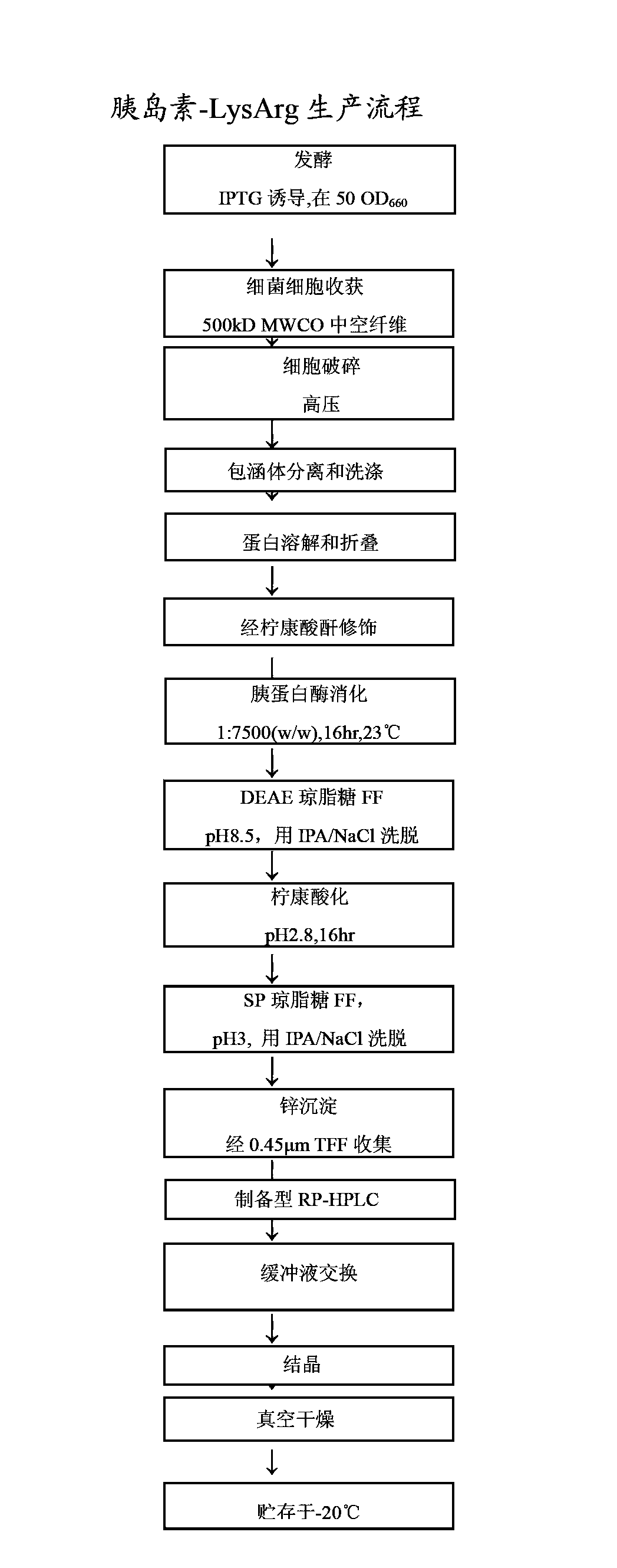
[0091] Ways to Produce Insulin Efficiently
[0092] expression cloning
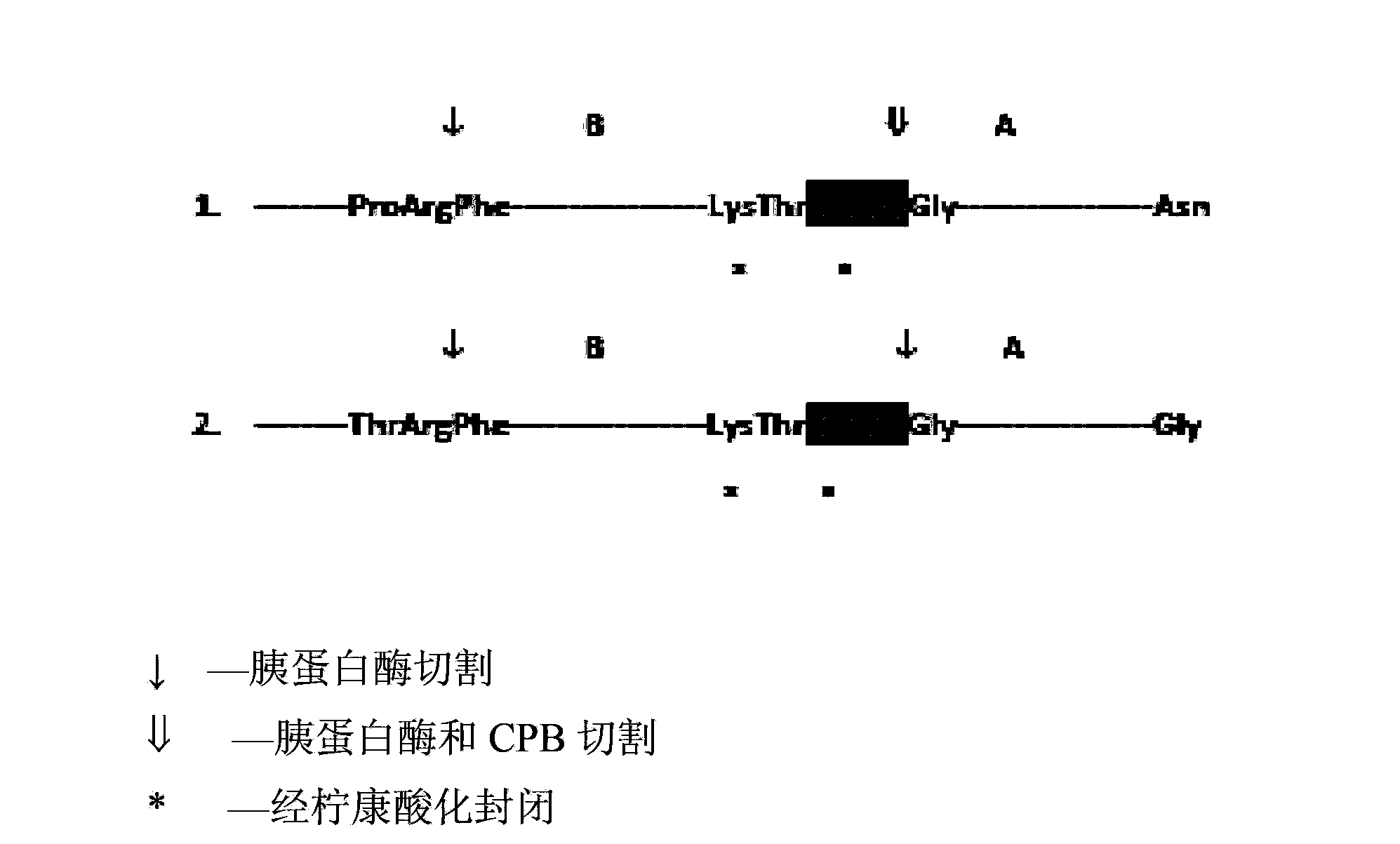
[0093] pass software to optimize the nucleotide sequence encoding the insulin precursor protein for improved expression in Escherichia coli. Synthetic genes were then assembled from the synthetic oligonucleotides and cloned into pET24a (Novagen) using NdeI and BlpI restriction enzyme sites. Escherichia coli BL21 strain (DE3) cells were transfected with the above clones. The gene is transcribed under the control of the T7 promoter and lac operator. Transcription termination is controlled by the T7 terminator and translation initiation is initiated by the pET24 ribosome binding site. Expression of the 12.7 kD fusion protein serving as an insulin precursor was induced by IPTG, resulting in very high levels of insulin precursor. This precursor contains a Cu / Zn SOD moiety as the amino-terminal moiety fused to a proinsulin-like moiety via an Arg residue, where Lys-Arg links the B and A chains of insulin. ...
Embodiment 2
[0106] Efficiently produced insulin is active in rats
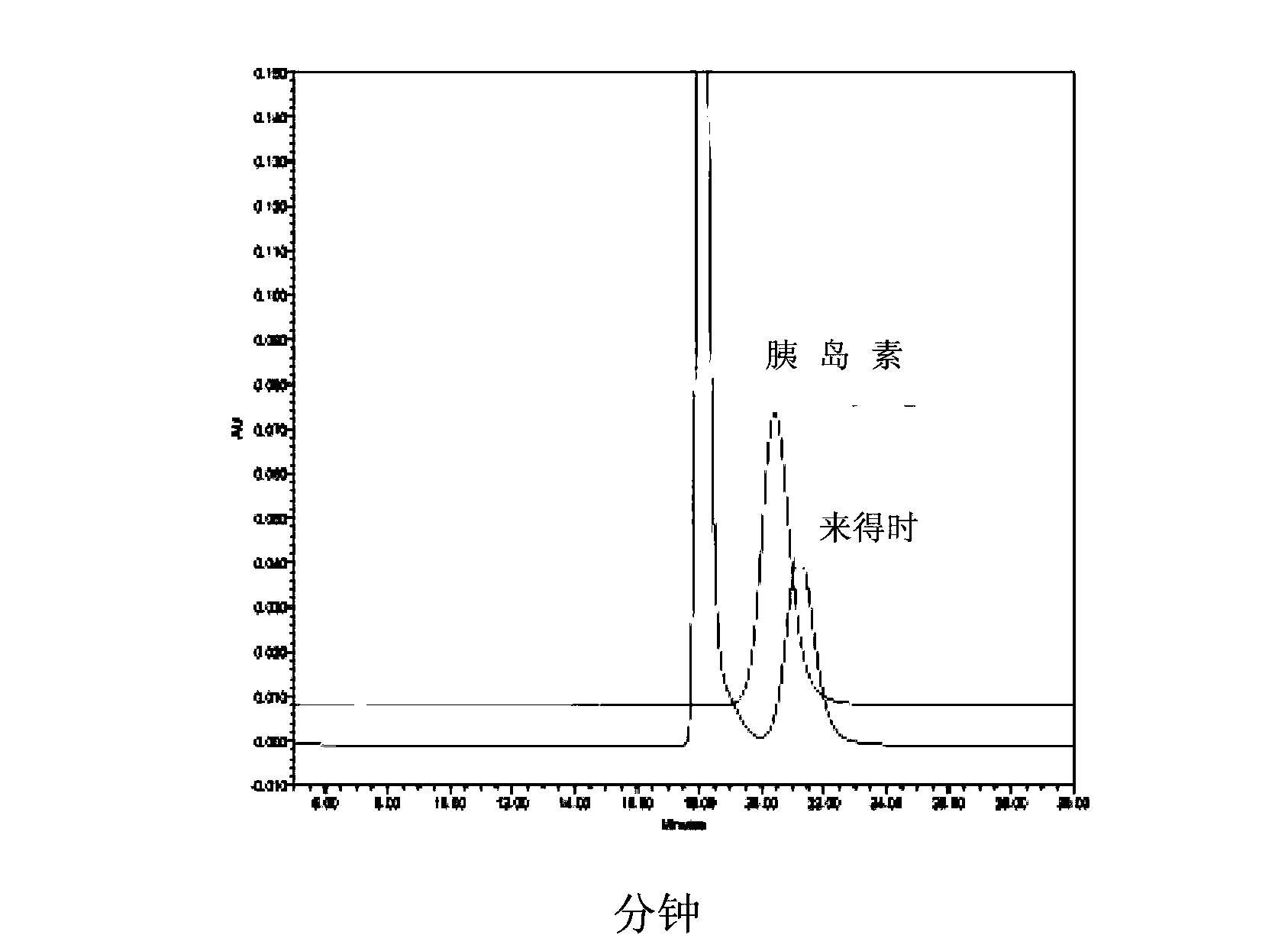
[0107] In order to evaluate the potential therapeutic effect of the long-acting insulin analog produced in Example 1, in streptozotocin (STZ)-induced diabetic rats by measuring their blood glucose levels at different time points after administration, test Insulin - LysArg and Lantus (Junod et al., 1969). Young Sprague-Dawley male rats, approximately 9 weeks of age, received a single intraperitoneal injection of 60 mg STZ / kg body weight. Five days after induction of diabetes, most animals exhibited blood glucose values above 300 mg / dL. The animals received the test material equivalent to 6 IU / kg by subcutaneous injection on 3 consecutive days. The study consisted of 3 experimental groups of 6 animals each, treated with the 2 different insulins specified above and vehicle as control. Blood glucose was measured before treatment and 1, 4, 8, and 24 hours after each dosing day. Such as Figure 5 As shown, both test mate...
Embodiment 3
[0109] Efficiently produced insulin with long-acting insulin activity in dogs
[0110] The effect of different insulin preparations on lowering blood glucose levels was tested in alloxan-induced diabetic beagle dogs. Eight healthy castrated female dogs, aged 2–5 years and weighing 10–17 kg, were injected intravenously with 50 mg / kg body weight of alloxan. Three days after treatment, all dogs developed a reproducible experimental diabetic state, showing hyperglycemia and other characteristic symptoms (Watanabe et al., The pathological features of alloxan diabetes in beagle dogs, J Toxicol Pathol, 2004, 17, 187- 195).
[0111] Insulin-LysArg ("Insulin KR") prepared as described above was compared with two commercially available insulin formulations, Humulin R (Elli Lily) and Lantus (Sanofi-Aventis). The three formulations and the vehicle control were tested in a randomized crossover study in 8 diabetic beagle dogs.
[0112] Different insulin preparations and the control were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com