Mfg-e8 and uses thereof
A technology of MFG-E8 and hmfg-e8, applied in the field of MFG-E8 and its application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0097] Embodiment 1. The treatment of recombinant human MFG-E8 and sepsis
[0098] Materials and methods
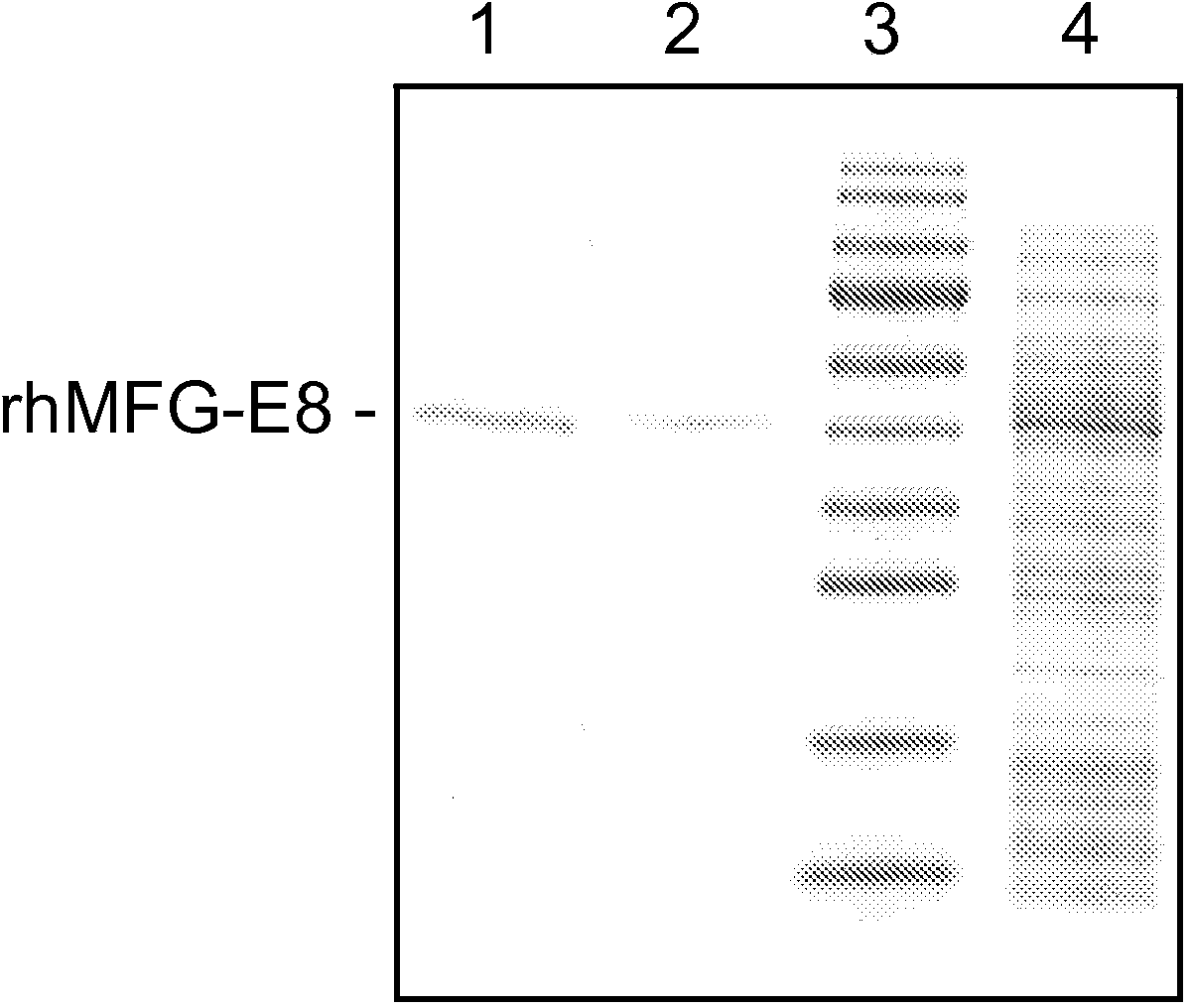
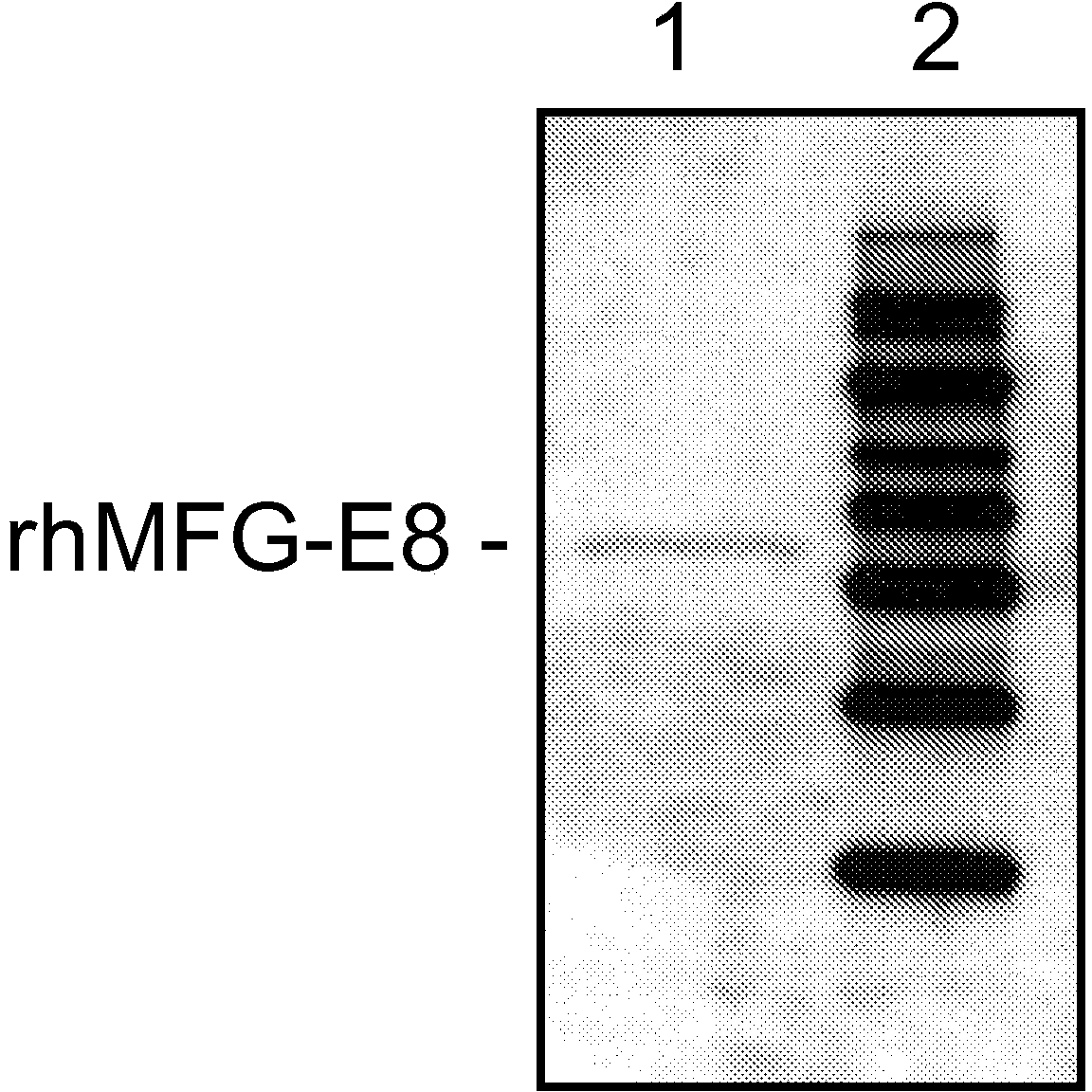
[0099] Expression of recombinant human MFG-E8: Amplify the plasmid template containing human MFG-E8 cDNA by polymerase chain reaction to obtain a 1095bp fragment encoding the mature region of human MFG-E8 (364 amino acids, R24-R387, SwissProt#:Q08431) (SEQ ID NO: 3). The open reading frame was cloned into the SalI and NotI sites of the pET-28a(+) vector (Novagen, Madison, WI), downstream of the phage T7 RNA polymerase promoter. The final protein product contains 6 histidines fused to the N-terminus of human MFG-E8. The plasmid was transformed into E. coli BL21(DE3) cells. Cells were grown overnight at 37°C in 2YT medium (Invitrogen) containing kanamycin. rhMFG-E8 protein production was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 1.0 mM, and the cells were grown for an additional 5 h at 25°C. Cells were harvested b...
Embodiment II
[0119] Embodiment II. Use recombinant human MFG-E8 to treat cerebral ischemia
[0120] Materials and methods
[0121] Experimental Animals: Male Sprague-Dawley rats (300-350 g) purchased from Charles River Laboratories (Wilmington, MA) were used in this study. Rats were housed under standard conditions (room temperature, 22°C, 12 / 12-h light / dark cycle) with regular access to standard Purina rat chow and water. Animals were acclimated to these conditions for at least 5 days before being used in experiments. All animal experiments were performed in accordance with the National Institutes of Health guidelines for laboratory animals. The protocol was approved by the Institutional Animal Use and Care Committee (IACUC) of the Feinstein Institute for Medical Research.
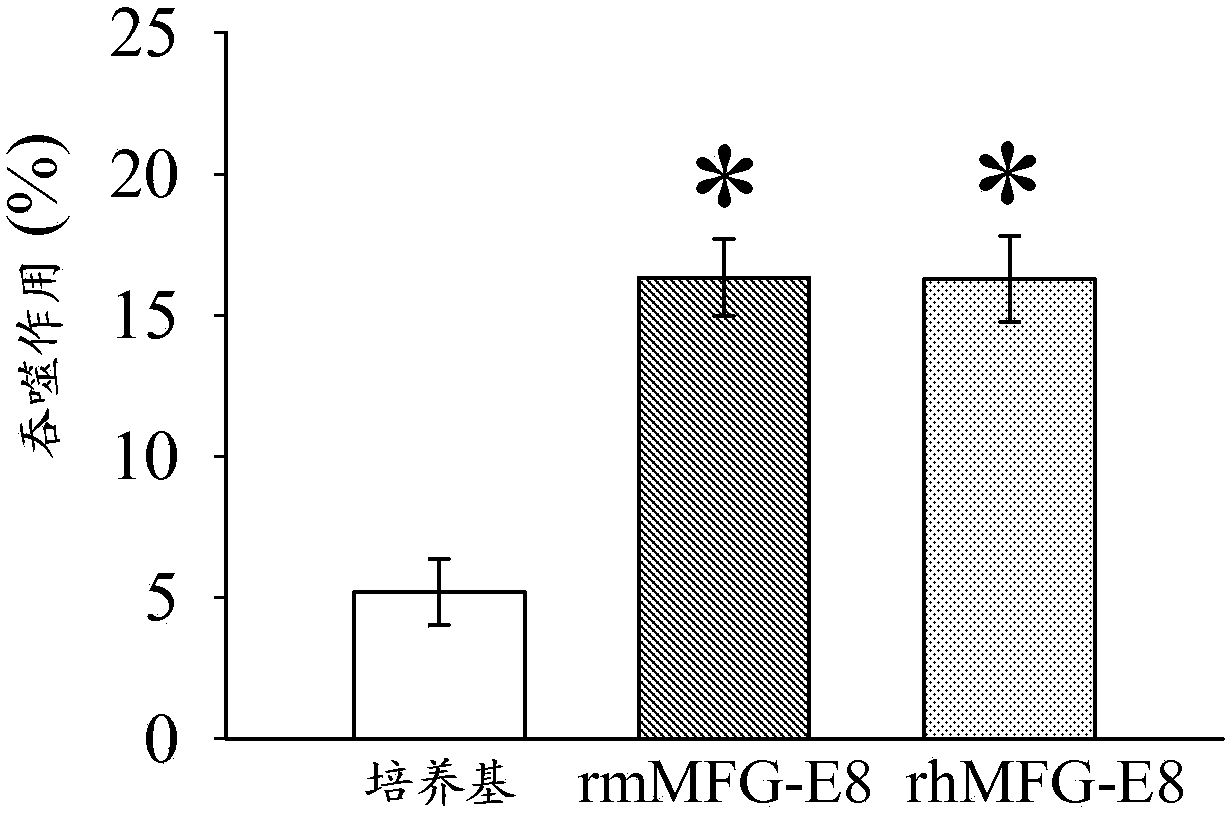
[0122]Cerebral ischemia model: Prior to induction of cerebral ischemia, rats were fasted overnight but had access to water ad libitum. Permanent focal cerebral ischemia was induced by middle cerebral artery occlus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com