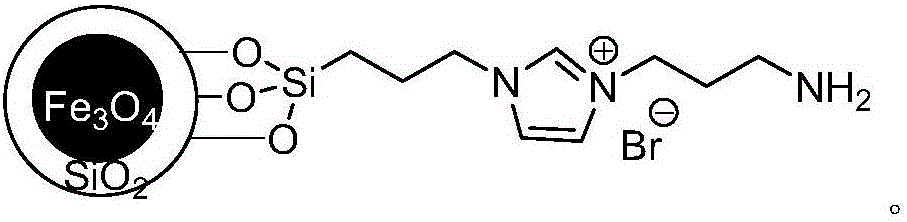
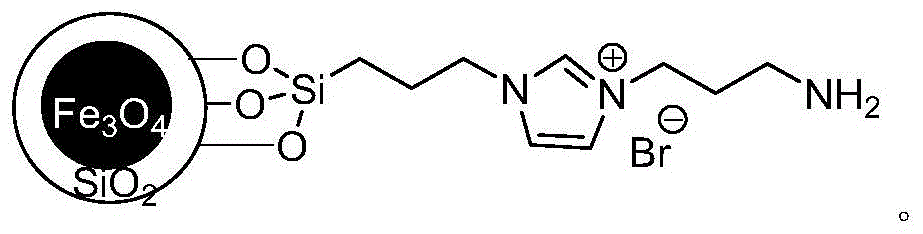
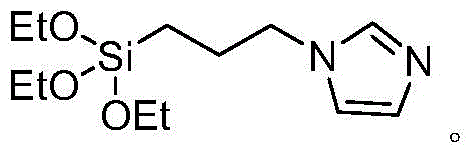
A kind of method for preparing diaromatic ring substituted olefin
An aromatic ring and aromatic technology, which is applied in the preparation of carboxylic acid nitriles, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of a large number of catalysts, harsh reaction conditions, catalyst recovery and mechanical application, and large-scale promotion and use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Thiphenaldehyde (5mmol), 2-cyanomethylbenzimidazole (5mmol), 0.735g catalyst, and water (10mL) were sequentially added into a 50mL three-neck flask, stirred at room temperature for 2 hours, and detected by TLC, the raw materials basically disappeared. The catalyst was collected, the reaction solution was poured out, filtered, and the obtained solid was recrystallized with ethanol to obtain the product with a yield of 79% and a content of 98%.
[0025] 2-(1H-benzoimidazol-2-yl)-3-(thiophen-2-yl)acrylonitrile: 1 H NMR (400MHz, CDCl 3 ):δ7.15-7.22(m,1H),7.30-7.37(m,2H),7.50-7.52(m,1H),7.66-7.70(m,2H),7.76-7.77(m,1H),7.87 -7.89(m,1H),8.63(s,1H); 13 CNMR (100MHz, CDCl 3 ): δ98.1, 115.6, 115.7, 116.7, 123.6, 129.1, 134.5, 136.8, 137.0, 139.3, 147.6
Embodiment 2
[0027] Thiphenaldehyde (5mmol), 2-cyanomethylbenzimidazole (5mmol), 0.147g catalyst, and water (10mL) were sequentially added into a 50mL three-neck flask, stirred at room temperature for 5 hours, and detected by TLC, the raw materials basically disappeared, and an external magnetic field was applied to absorb The catalyst was collected, the reaction solution was poured out, filtered, and the obtained solid was recrystallized with ethanol to obtain the product with a yield of 62% and a content of 97%.
Embodiment 3
[0029] Thiphenaldehyde (5mmol), 2-cyanomethylbenzimidazole (6mmol), 0.147g catalyst, and water (10mL) were sequentially added into a 50mL three-neck flask, stirred at room temperature for 3 hours, and detected by TLC, the raw materials basically disappeared, and an external magnetic field was applied to absorb The catalyst was collected, the reaction solution was poured out, filtered, and the obtained solid was recrystallized with ethanol to obtain the product with a yield of 76% and a content of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com