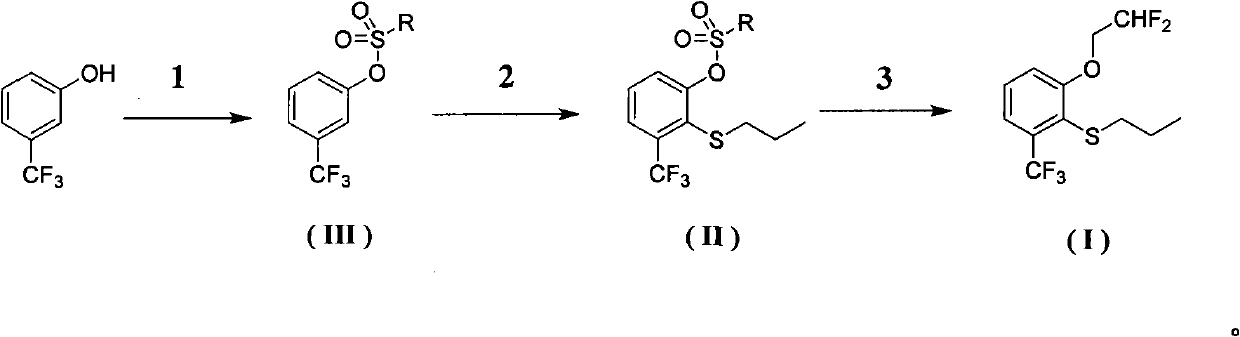
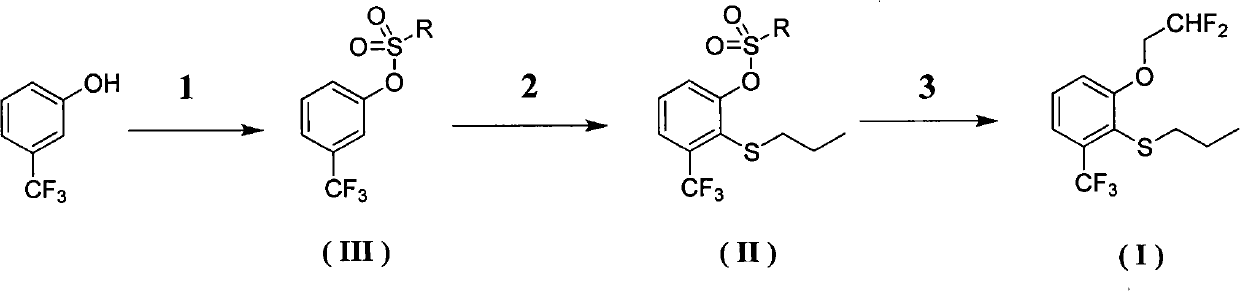
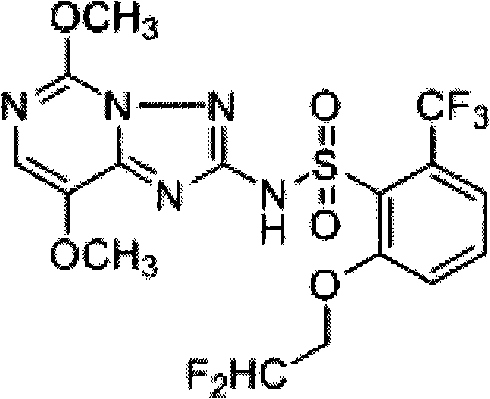
Preparation method of 2-(2', 2'-difluoroethoxyl)-6-trifluoromethyl phenyl propyl sulfide
A technology of trifluoromethylphenylpropyl sulfide and difluoroethoxy, applied in 2-(2',2'-difluoroethoxy)-6-trifluoromethylphenylpropylsulfide In the field of ether preparation, it can solve the problems of adding reaction steps to protective groups, not being able to meet industrial production, and increasing costs, and achieve the effects of improving synthesis effects, reducing production costs, and simplifying reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Synthesis of compound Ⅲ-1
[0039]
[0040] Take a 250ml three-necked bottle with a stirring bar inside. Protected with nitrogen, add 0.1234g of triethylamine hydrochloride, add 21g of triethylamine, stir, add 100ml of dichloromethane. Then 7.86 g of m-trifluoromethylphenol were added. Weigh 9.13g of p-toluenesulfonyl chloride, add it into the reactor, stir, and track the reaction with a plate. Stir overnight. After the reaction, 50ml of water was added and stirred for 2h. Extraction was carried out with ethyl acetate and water, and the organic layer was washed successively with 2N hydrochloric acid, saturated aqueous sodium bicarbonate solution, and water twice, and the aqueous layer was removed. Afterwards, it was dried with anhydrous sodium sulfate, and the solvent was recovered under reduced pressure. 14.5 g of white solid was obtained.
[0041] The solid obtained in the previous step was a crude product, which was further purified by recrystallization ...
Embodiment 2
[0049] (1) Synthesis of compound Ⅲ-2
[0050]
[0051] Take a 250ml three-necked bottle with a stirring bar inside. Protected with nitrogen. At 0° C., 52 g of m-trifluoromethylphenol was dissolved in 150 ml of pyridine (this was a solvent), and then 95.2 g of trifluoromethanesulfonic anhydride was slowly added dropwise with stirring. After the addition was complete, it was stirred at this temperature for 0.5 hours, then warmed to room temperature, and the solution became dark. Stir overnight. After the reaction, 50ml of water was added and stirred for 2h. Partitioned with dichloromethane and water, the organic layer was washed successively with 2N hydrochloric acid, saturated aqueous sodium bicarbonate solution, and water twice, and the aqueous layer was removed. Afterwards, it was dried with anhydrous sodium sulfate, and the solvent was recovered under reduced pressure. Obtained 88.5 g of yellow oil. The crude product obtained in the previous step was further purified...
Embodiment 3
[0059] (1) Synthesis of compound a
[0060]
[0061] In the 2L reaction flask that is equipped with stirring and thermometer, the compound I of 130g (0.396moles) is dissolved in the 88% formic acid of 600ml, and chlorine gas is passed in under stirring with the speed of 2 grams / minute, and the temperature of reaction solution is controlled At 37 DEG C, the feeding amount of chlorine is 70% of compound (I) molar weight, after reaction will carry out 1.5 hours, temperature of reaction is down to 23 DEG C, the aqueous solution of sodium bisulfite of 63g11% is added reaction solution, has A white solid was produced, and at 5°C, 250ml of water was added to the mixed solution, fully precipitated, filtered, and the obtained white solid solution was ethyl acetate, dried over anhydrous sodium sulfate. The solvent was evaporated to obtain 135 g of white crystalline powder with a yield of 95% and a purity of 95%, which could be directly used in the next reaction. 1 H-NMR (400MHz, CDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com