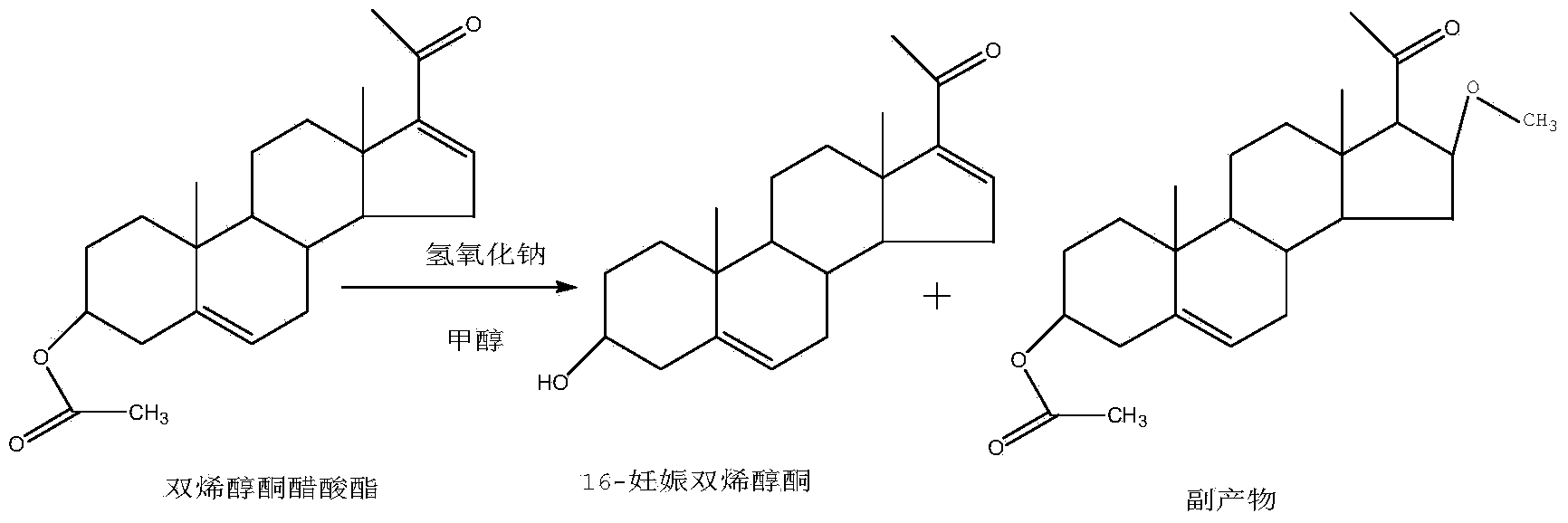
Method for synthesizing 16-dehydropregnenolone by taking dehydropregnenolone acetate as raw material
A technology of pregnant dienolone and diketene acetate, which is applied in the field of synthesis of chemical drugs, can solve problems such as serious side reactions, impact on product quality and yield, and damage, and achieve the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 20 grams of dienolone acetate and 200 milliliters of tetrahydrofuran (THF) in the reaction flask, stir for 10 minutes to dissolve;
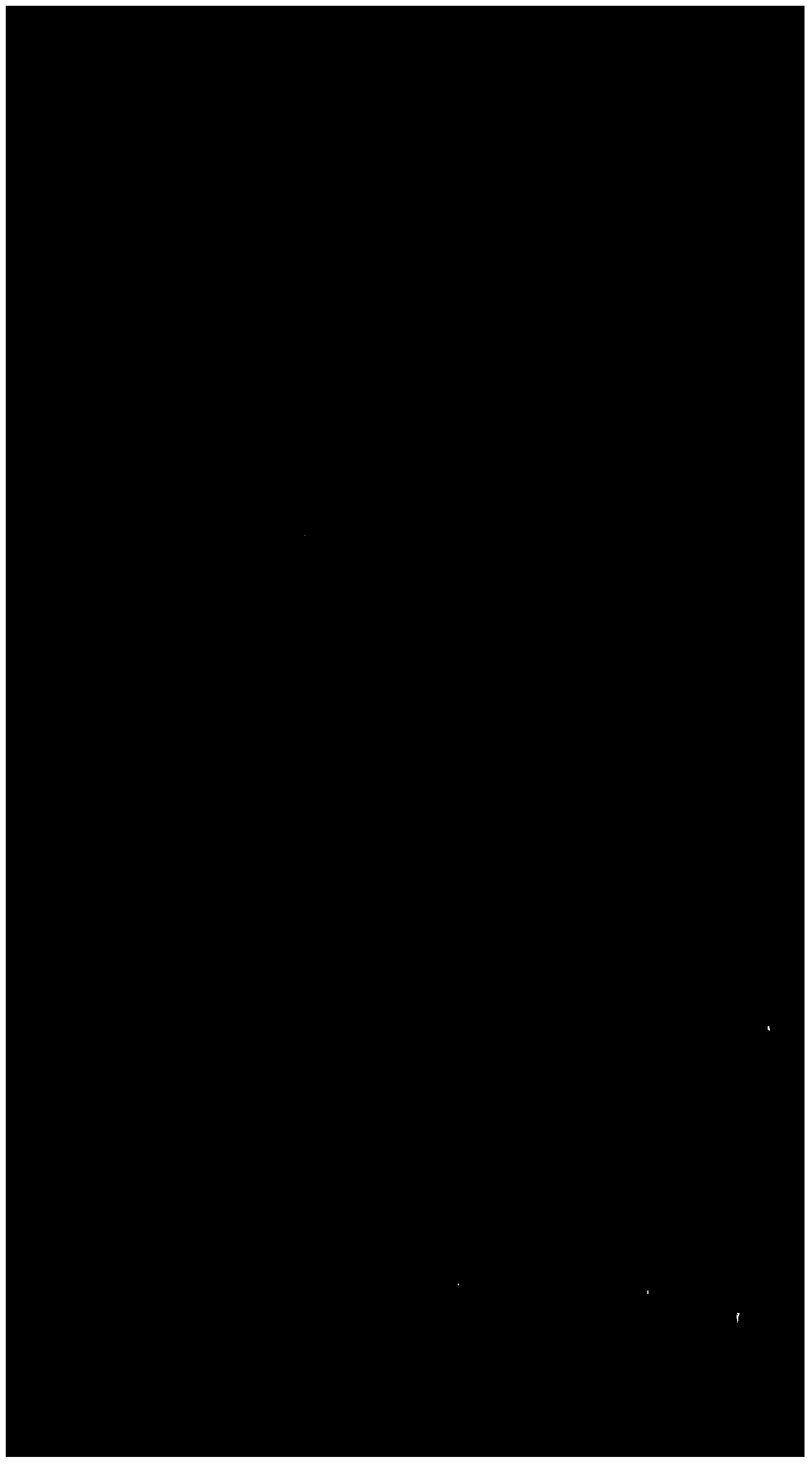
[0016] Then add 20 milliliters of 20% sodium hydroxide solution to the reaction flask, heat up to 20-30° C., stir the reaction and keep it warm for 1 hour, and then do TLC detection for the reaction. figure 1 A column is the point of contrast dienolone acetate, B column is the result of the spot plate of the reaction solution of this embodiment, B1 is the point of raw material dienolone acetate, and B2 is the product. When TLC detects that dienolone acetate disappears Add 10 ml of hydrochloric acid, adjust the pH to be neutral, raise the temperature to reflux temperature under normal pressure to recover most of the solvent, and recover the remaining solvent by rotary evaporation under reduced pressure; then add 200 ml of water to the residue of rotary evaporation, filter, and wash the filter cake with 100 ml of water , and dried to obt...
Embodiment 2
[0023] Add 10 grams of dienolone acetate to 100 milliliters of N,N-dimethylformamide (DMF) in the reaction flask, stir for 10 minutes to dissolve;
[0024] Add 10 milliliters of 20% sodium hydroxide solution, heat up to 20-30° C., and keep the temperature for 1 hour. After TLC detects that dienolone acetate disappears, add 5 milliliters of hydrochloric acid, and the dot plate diagram and figure 1 The same by-product point was not seen. Adjust the pH to be neutral, recover most of the solvent by raising the temperature under normal pressure, and recover the remaining solvent under reduced pressure; then add 200 ml of water, filter, wash the filter cake with 100 ml of water, and dry to obtain 8.5 g of 16-pregnant dienolone. Yield 91%.
Embodiment 3
[0026] Add 50 grams of dienolone acetate and 500 milliliters of dioxane in the reaction flask, stir and dissolve;
[0027] Add 50 milliliters of 20% sodium hydroxide solution, react at room temperature for 3 hours, TLC detects that dienolone acetate disappears, and the dot plate diagram and figure 1 The same by-product point is not seen, add 25 milliliters of hydrochloric acid, adjust the pH to be neutral, recover most of the solvent by increasing the temperature under normal pressure, and recover the remaining solvent under reduced pressure; then add 400 milliliters of water, filter, wash the filter cake with 100 milliliters of water, and dry to obtain 16-Pregnancy Dienolone 46g. Yield 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com