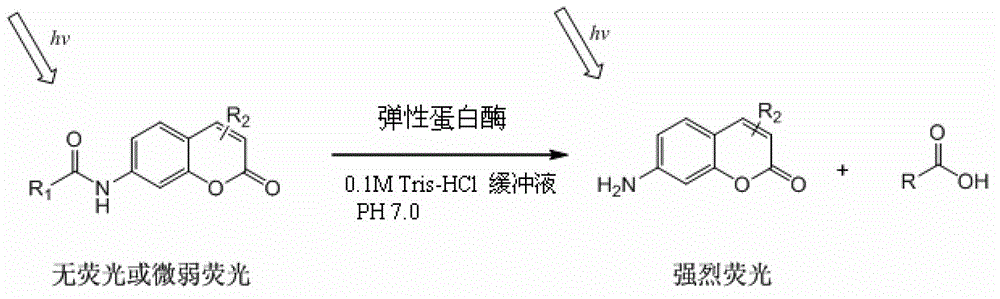
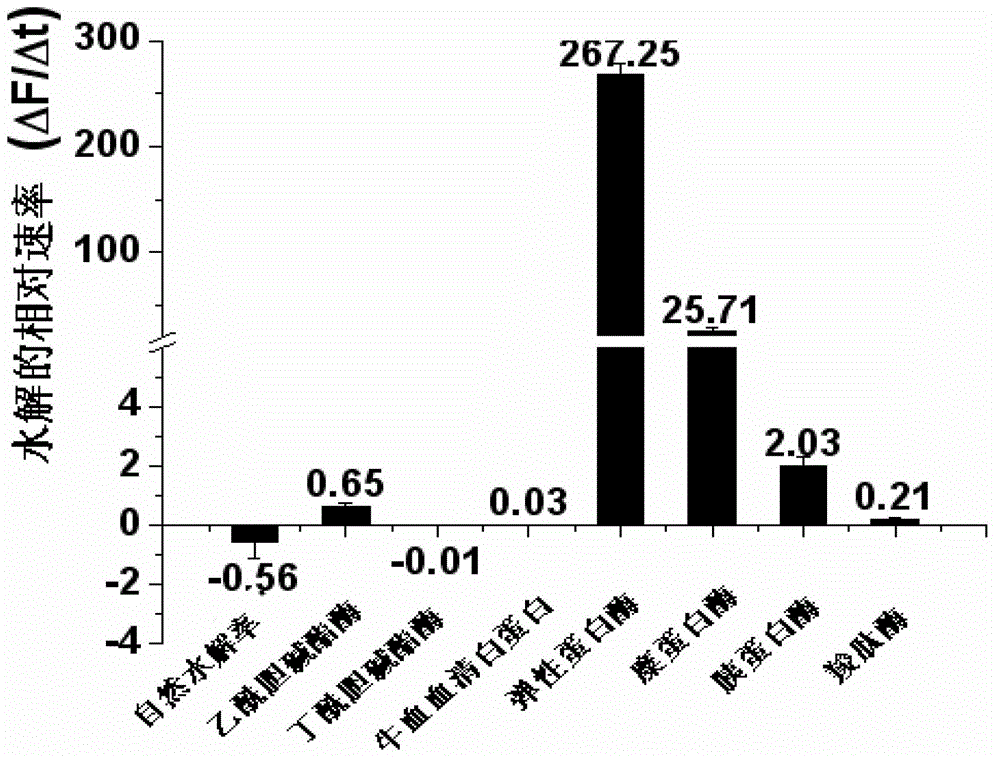
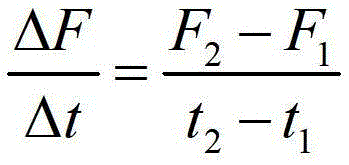Coumarin derivatives, preparation method of coumarin derivatives, and elastase activity detection method and kit
A technology of coumarin derivatives and elastase, which is applied in the application field of activity, can solve the problem of high-throughput screening of elastase inhibitors or accelerators, tedious high-performance liquid chromatography experiment process, storage and Harsh transportation conditions and other issues, to achieve the effect of low price, high atomic utilization rate, and high detection effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the coumarin derivative of the present invention comprises: under the condition of nucleophilic reaction and the presence of an acid-binding agent, in an organic solvent, the compound represented by formula (2) is contacted with one of acid chloride or acid anhydride;
[0027] Formula (2)
[0028] Wherein, the acid chloride is selected from pentafluoropropionyl chloride, one of heptafluorobutyryl chloride, preferably, the acid chloride is pentafluoropropionyl chloride; the acid anhydride is selected from pentafluoropropionic anhydride, heptafluorobutyric anhydride One, preferably, the acid anhydride is pentafluoropropionic anhydride.
[0029] In the preparation method of the coumarin derivatives described in the present invention, the nucleophilic reaction is a reaction between the amino group and various substituted acid chlorides and acid anhydrides, and the conditions of the nucleophilic reaction include a temperature of minus 5 to 30°C, a...
Embodiment 1
[0071] This example is used to illustrate the preparation method of the coumarin derivatives of the present invention.
[0072] In an organic solvent (dichloromethane, 20ml), in the presence of an acid-binding agent (pyridine, 2.5mmol), the compound (7-amino-4-trifluoromethylcoumarin, 1mmol) represented by formula (2) ) and acid anhydride (pentafluoropropionic anhydride, 2 mmol) at 5° C. for 30 minutes to obtain a contacted material. Then the contacted material was washed with water (50ml) and saturated citric acid solution (50ml) in sequence to obtain the washed material. The oil phase in the washed material was separated and dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was purified by a silica gel column to obtain 0.15 g of a white solid product (1).
[0073] Detect this white solid product with NMR and MS, data is 1 H NMR (600MHz, DMSO-d 6 ): δppm: 7.03(s,1H), 7.79(s,2H), 7.88(s,1H), 11.82(s,1H)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com