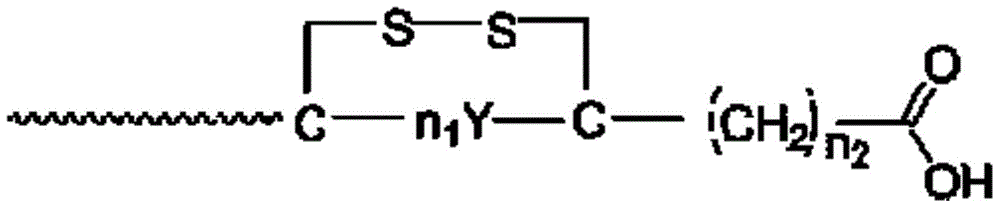Long-acting intestinal hormone polypeptide analog and application for treating type 2 diabetes
A technology of analogs and drugs, applied in the field of pharmaceuticals, can solve the problems of short half-life of liraglutide, inconvenient clinical use, and failure to meet clinical standards, and achieve the effect of facilitating clinical promotion and application and prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the solid-phase synthesis of polypeptide
[0040] Using the solid-phase polypeptide synthesis method of the Fmoc strategy, the CS336X instrument produced by CSBio Company was used to synthesize the polypeptide of the present invention. The method of synthesis was carried out according to the manufacturer's instruction manual. The "Fmoc strategy" described herein refers to a synthetic method for synthesizing polypeptides by sequentially condensing amino-terminal Fmoc-protected amino acids in the presence of coupling reagents using polymer resin as a solid-phase reaction substrate. For its specific method, see Fmoc solid phase peptide synthesis: a practical approach, 2000, Oxford University Press.
[0041] The prepared polypeptide was purified using HPLC C18 semi-preparative column, and the mobile phase was acetonitrile. The polypeptide freeze-dried powder is obtained by desalting and freeze-drying. The polypeptides included in the patent of the present inv...
Embodiment 2
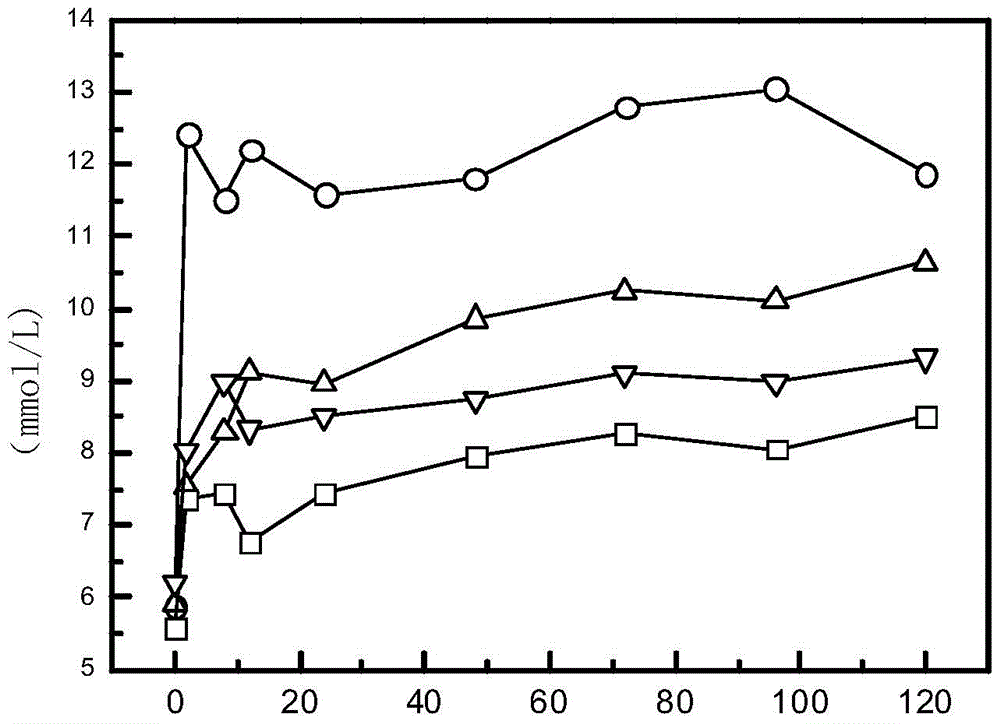
[0042] Example 2: Hypoglycemic Function of GLP-1 Analogs
[0043] In this embodiment, the polypeptides used are as follows:
[0044] SEQ ID NO 4: HAEGTFTSDVSSYLEGQAAKEFIAWLCKGRGGC—aminocaproic acid;
[0045] SEQ ID NO 5: HAEGTFTSDVSSYLEGQAAK EFIAWLVKCRGAAC—amino caprylic acid;
[0046] SEQ ID NO 6: HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRCVVVVVC—aminodecanoic acid
[0047] Dissolve 1 mg of each of the above polypeptides in 1 ml of normal saline to make a polypeptide solution, and inject the polypeptide solution subcutaneously into mice (200 μl / mouse, 6 mice / group, purchased from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences). After 30 minutes of administration, 400 Micrograms of glucose were injected into each mouse. The blood glucose of the mice was measured at 2 hours, 24 hours, 48 hours, 72 hours and 96 hours after the glucose injection (note: the same dose of glucose was given again two hours before each blood glucose measurement), and the results w...
Embodiment 3
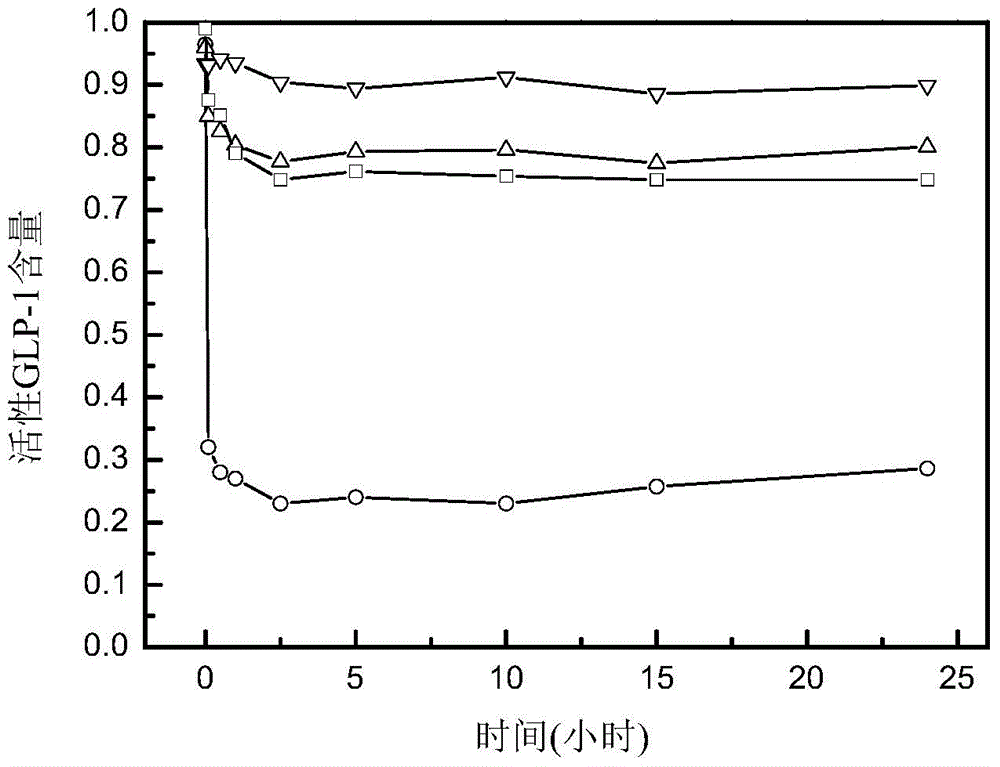
[0049] Example 3: Stability determination of GLP-1 analogs in human serum
[0050] In this embodiment, the polypeptides used are as follows:
[0051] SEQ ID NO 4: HAEGTFTSDVSSYLEGQAAKEFIAWLCKGRGGC—aminocaproic acid;
[0052] SEQ ID NO 5: HAEGTFTSDVSSYLEGQAAK EFIAWLVKCRGAAC—amino caprylic acid;
[0053] SEQ ID NO 6: HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRCVVVVVC—aminodecanoic acid
[0054] (1) Serum preparation: Three blood samples were taken from volunteers with a vacuum blood collection needle (BD Biosciences, Franklin Lakes, NJ), and then immediately centrifuged at 13,000 rpm for 20 minutes on a centrifuge, and the upper layer of serum was taken for later use.
[0055] (2) Dissolve 0.1 mg of each of the above three polypeptides and GLP-1 standard (purchased from Shanghai Shenggong Company, its sequence is: HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG) in 0.5 ml of normal saline, and add to 1 ml of rat serum after fully dissolving ( Purchased from Invitrogen Company), the serum was marked as bl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com