A kind of icatibant injection composition and its preparation method and preparation
A technology for icatibant and injection, applied in the field of medicine, can solve the problems of high production cost, complex production line, high product price, etc., and achieve the effect of maintaining stability and excipient safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
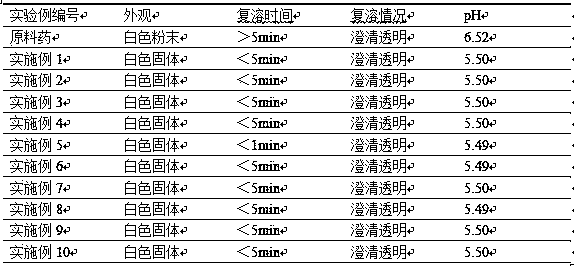
[0041] The preparation of embodiment 1 icatibant injection composition
[0042] Weigh 30.0mg of icatibant, 60.0mg of lyoprotectant (mannitol) and 60.0mg of solubilizer and stabilizer (glucose) in a sterile container, stir and dissolve the mixture with 1mL of water for injection, after stirring and dissolving, wash with acetic acid Adjust the pH to 5.5, continue to dilute to 3 mL with water for injection, filter with a 0.22 μm microporous membrane under sterile conditions, freeze-dry, and obtain the product.
[0043] The freeze-drying process is as follows: ① In the pre-freezing stage, the sample reaches the set temperature of -25°C within half an hour and maintains it for 2 hours, and then the sample reaches the pre-freezing temperature of -55°C within half an hour and maintains it for 2 hours; ② Freeze sublimation In the primary drying stage, the sample reaches the set temperature of -10°C within 1 minute and maintains it for 25 hours; ③ in the freeze-sublimation secondary dr...
Embodiment 2
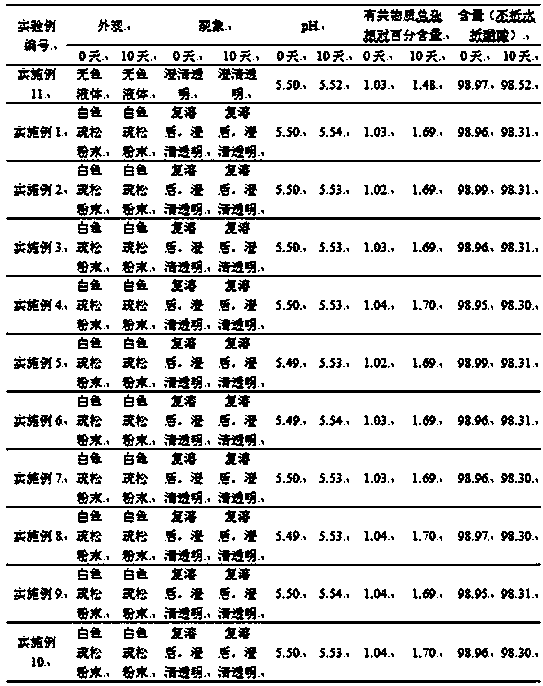
[0044] The preparation of embodiment 2 icatibant injection composition
[0045] Weigh 30.0mg of icatibant, 60.0mg of lyoprotectant (glucose) and 60.0mg of solubilizer and stabilizer (sucrose) into a sterile container, stir and dissolve the mixture with 1mL of water for injection, after stirring and dissolving, adjust with acetic acid The pH value is 5.5, continue to dilute to 3 mL with water for injection, filter with a 0.22 μm microporous membrane under sterile conditions, and freeze-dry to obtain the obtained product.
[0046] The freeze-drying process is as follows: ① In the pre-freezing stage, the sample reaches the set temperature of -25°C within half an hour and maintains it for 2 hours, and then the sample reaches the pre-freezing temperature of -55°C within half an hour and maintains it for 2 hours; ② Freeze sublimation In the primary drying stage, the sample reaches the set temperature of -10°C within 1 minute and maintains it for 25 hours; ③ in the freeze-sublimation...
Embodiment 3
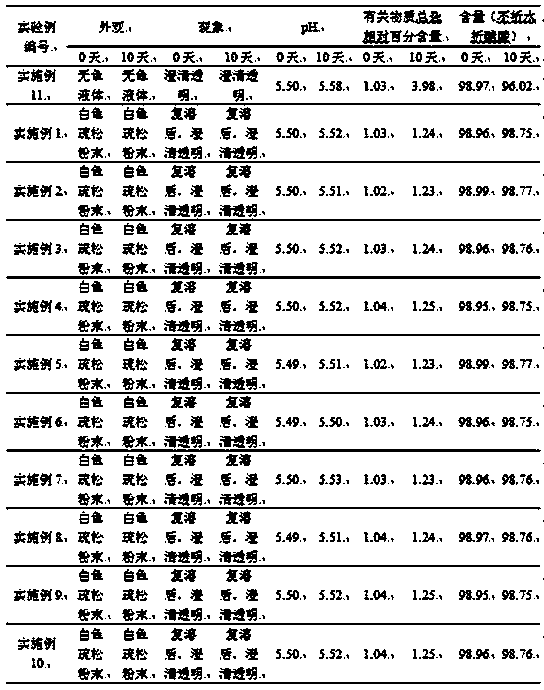
[0047] The preparation of embodiment 3 icatibant injection composition
[0048] Weigh 30.0mg of icatibant, 60.0mg of lyoprotectant (sucrose) and 60.0mg of solubilizer and stabilizer (glycerin) into a sterile container, stir and dissolve the mixture with 1mL of water for injection, after stirring and dissolving, adjust with acetic acid The pH value is 5.5, continue to dilute to 3 mL with water for injection, filter with a 0.22 μm microporous membrane under sterile conditions, and freeze-dry to obtain the obtained product.
[0049] The freeze-drying process is as follows: ① In the pre-freezing stage, the sample reaches the set temperature of -25°C within half an hour and maintains it for 2 hours, and then the sample reaches the pre-freezing temperature of -55°C within half an hour and maintains it for 2 hours; ② Freeze sublimation In the primary drying stage, the sample reaches the set temperature of -10°C within 1 minute and maintains it for 25 hours; ③ in the freeze-sublimatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com