Method for preparing methyl cedryl ether from Chinese fir oil
A technology of methyl cedarwood ether and fir oil, which is applied in the direction of ester reaction to prepare ether, ether preparation, metal alcohol preparation, etc. It can solve the problems of rot, waste of resources, and not being well utilized, so as to achieve pure aroma and avoid bumping The effect of punching and coking and shortening the residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
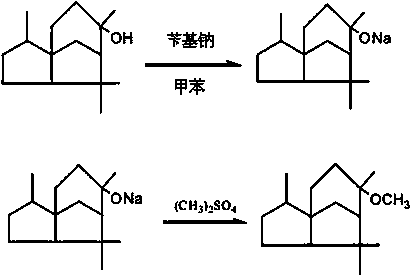
[0034] In a reaction kettle with a stirring and reflux condenser, add 170ml of toluene and 137g of benzyl sodium, heat to reflux under stirring, add dropwise a solution of 223g of cetaxel and 200ml of toluene, control the temperature of the reaction system to reflux, drop The addition time was 30 minutes, and after the dropwise addition was completed, the reaction was refluxed for 1.5 hours, then cooled to room temperature, and the first step reaction was completed.
[0035] Continue to add 122g of dimethyl sulfate dropwise, and the dropwise addition is completed within 30min. After the dropwise addition, react at 70°C for 2.5h, wash the reaction mixture with 100ml of 10% sodium bicarbonate solution, let stand to separate layers, and separate the water layer , the oil layer was washed with 10% aqueous sodium chloride solution until neutral. Atmospheric distillation first recovers the solvent toluene, and then conducts rectification.
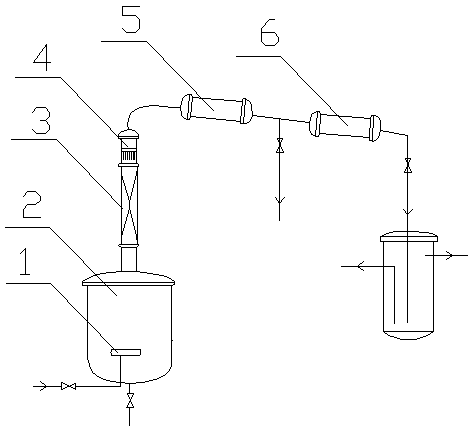
[0036] A superheated steam conduit is arr...
Embodiment 2
[0040]In a reaction kettle with a stirring and reflux condenser, add 195ml of toluene and 182.4g of benzyl sodium, heat to reflux under stirring, add dropwise a solution of 222.4g of cetaxel and 320 ml of toluene, and control the temperature of the reaction system to reflux , the dropwise addition time is 30min, after the dropwise addition is completed, reflux for 1.5h, then cool to room temperature, and the first step reaction is completed.
[0041] Continue to add 151.4g of dimethyl sulfate dropwise, and the dropwise addition is completed within 30min. After the dropwise addition, react at 65°C for 2.5h, wash the reaction mixture with 100ml of 10% sodium bicarbonate solution, let stand to separate layers, and separate the water layer , the oil layer was washed with 10% aqueous sodium chloride solution until neutral. Atmospheric distillation first recovers the solvent toluene, and then conducts rectification.
[0042] A superheated steam conduit is arranged at the bottom of ...
Embodiment 3
[0046] In a reaction kettle with a stirring and reflux condenser, add 100ml of toluene and 114g of benzyl sodium, heat to reflux under stirring, add dropwise a solution of 223g of cetaxel and 250ml of toluene, control the temperature of the reaction system to reflux, drop The addition time was 30 minutes, and after the dropwise addition was completed, the reaction was refluxed for 1 hour, then cooled to room temperature, and the first step reaction was completed.
[0047] Continue to add 151.6g of dimethyl sulfate dropwise, and the dropwise addition is completed within 30min. After the dropwise addition, react at 80°C for 2.5h, wash the reaction mixture with 100ml of 10% sodium bicarbonate solution, let stand to separate layers, and separate the water layer , the oil layer was washed with 10% aqueous sodium chloride solution until neutral. Atmospheric distillation first recovers the solvent toluene, and then vacuum distillation. .
[0048] A superheated steam conduit is arra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com