Somatostatin receptor agonist polypeptide and its application
A somatostatin receptor and agonist technology, which is applied in the field of somatostatin receptor agonist polypeptides, can solve problems such as the development of somatostatin receptor agonist polypeptide liver cancer drugs, and achieve the prevention and treatment of liver cancer recurrence , Promote activity, improve the effect of survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
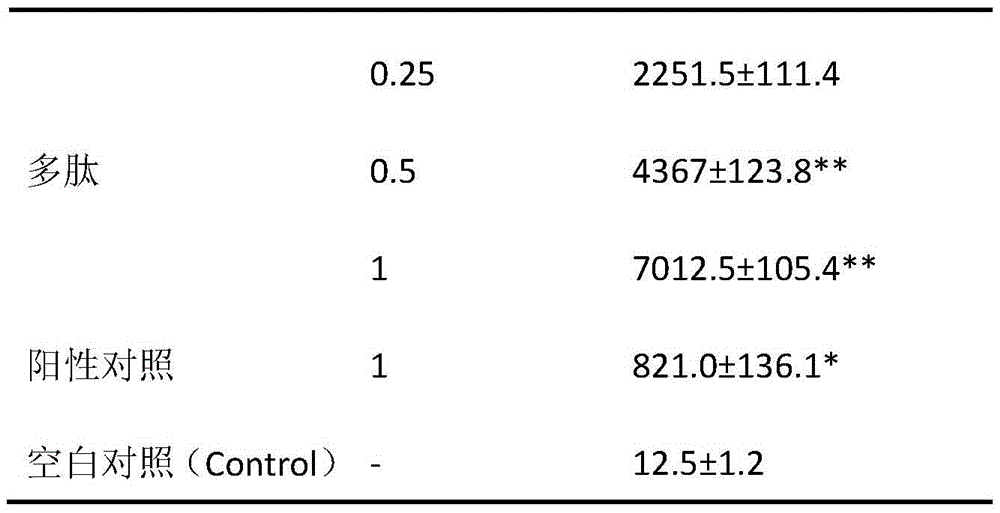
[0014] Effect of Somatostatin Receptor Agonist Peptides on the Affinity of Somatostatin Receptor and Somatostatin in Vitro.
[0015] HepG2 cells grown logarithmically at 1.0 x 10 5 Add it into a 96-well culture plate, cultivate for 24 hours, add different concentrations of the experimental drug somatostatin receptor agonist polypeptide 2 and the positive control drug vincristine in the experimental wells and positive drug control wells; add the same volume of solvent to the blank group, and simultaneously ,join in 125 I-labeled somatostatin. Five replicate wells were set up in each well, and cultured for 48 hours. Wash away the supernatant, calculate the radiation intensity with a γ-liquid scintillation counter, and judge the binding force between the somatostatin receptor and somatostatin. The stronger the radiation intensity, the more the binding. Conversely, the weaker the radiation intensity, the more binding. few. The results showed that as the concentration of somato...
Embodiment 2
[0020] IC50 of somatostatin receptor agonist polypeptide 2 on the growth and survival of tumor cells cultured in vitro.
[0021] The MTT colorimetric method was used. HepG2 cells with logarithmic growth were added to 96-well culture plate at 1.0×105 and cultured for 24 hours. Different concentrations of experimental drug somatostatin receptor agonist polypeptide 2 and positive control drug Changchun Neobase; add the same volume of solvent to the blank group. Set up five duplicate wells per well, culture for 48h, add MTT to each well at 0h, 2h, 8h, 14h, 20h, 24h, 36h, and 48h respectively, add DMSO after 4h of action, incubate for 30min, and incubate at 620nm in a microplate reader Measure the absorbance A value, according to the formula tumor cell growth inhibition rate=(1-absorbance value of the experimental group / absorbance value of the control group)×100%. The calculated IC50 of the experimental drug was 7.49 μM.
Embodiment 3
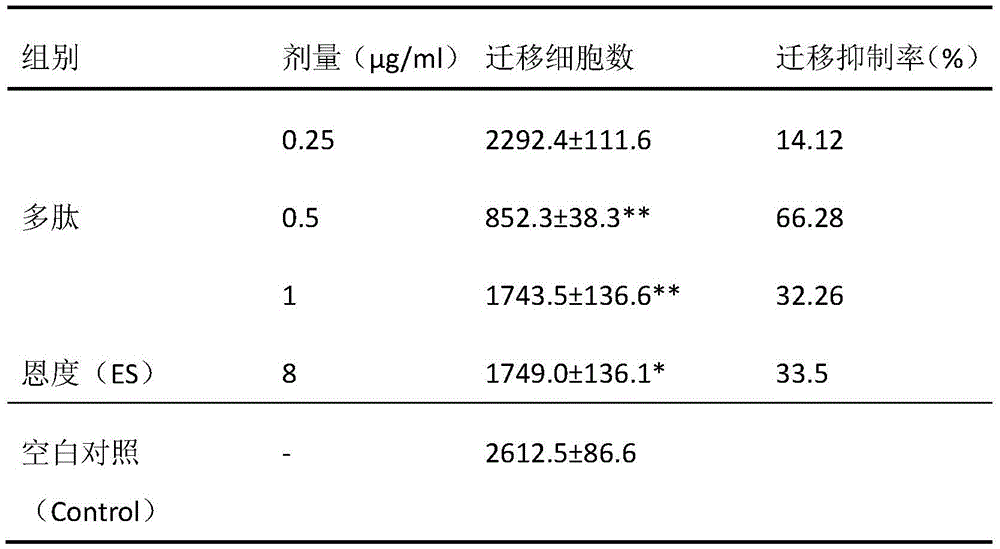
[0023] Effect of Somatostatin Receptor Agonist Peptides on HepG2 Cell Migration.
[0024] 10 mg / ml Matrigel (BD Company, USA) was diluted 1:3 with serum-free cell culture medium, coated on a Transwell chamber (Greiner Company, USA) membrane, and air-dried at room temperature. HepG2 cells cultured to the logarithmic growth phase were digested with trypsin, collected, resuspended in serum-free cell culture medium, counted under a microscope, and the cell concentration was adjusted to 1×105 cells / ml. Prepare each group of test liquids, and group them as follows: Blank control group: serum-free cell culture fluid without drugs; Endostar group: dilute 5 mg / ml of Endostar stock solution to Predetermined concentration; somatostatin receptor agonist polypeptide group: the somatostatin receptor agonist polypeptide is diluted to each predetermined concentration with drug-free serum-free cell culture medium. The cells were inoculated into the Transwell chamber, 100 μl per well, and each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com