Electrochemical cell
An electrochemical and battery technology, applied in the field of electrochemical batteries, can solve the problems of reduced ion conductivity, reduced charge and discharge capacity of electrochemical batteries, and degradation of cycle life characteristics, and achieves high reliability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] The positive electrode 30 can be prepared by currently known preparation methods.
[0099] For example, as the preparation method of the positive electrode 30, a method of mixing the positive electrode active material with an optional positive electrode conductive aid and / or positive electrode binder to form a positive electrode mixture, and forming the positive electrode mixture into an arbitrary shape such as a film . For example, a slurry may be prepared by mixing an arbitrary solvent with a positive electrode mixture, coated on the positive electrode current collector 31 , dried, and then compression-molded to form a slurry.
[0100] In addition, the positive electrode current collector 31 is not particularly limited, and conventionally known positive electrode current collectors can be used. For example, an aluminum foil made of aluminum alloy or pure aluminum is mentioned. In addition, the aluminum foil is preferably subjected to treatment to increase the specif...
Embodiment 1
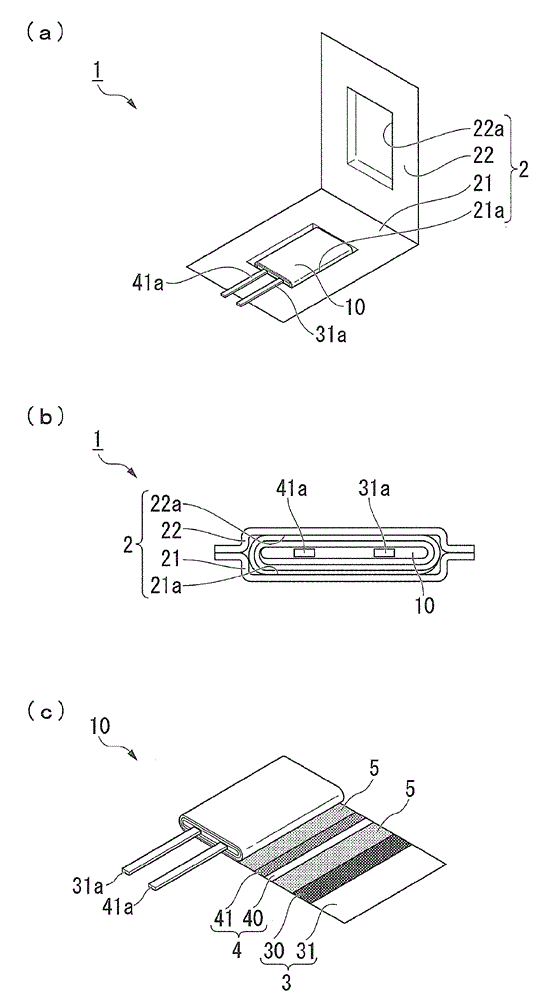
[0228] In Example 1, first, as an electrochemical cell, prepare as figure 1 (a)~(c) The laminate type nonaqueous electrolyte secondary battery shown.
[0229] In this example, if figure 1 As shown in (a), the outer package 2 is an aluminum laminated film in which a nylon layer (25 μm), an aluminum foil (40 μm), and a polypropylene layer (30 μm) are laminated in this order.
[0230] In addition, the negative electrode body 4 has a negative electrode 40 and a negative electrode current collector 41 . The negative electrode 40 is composed of a negative electrode active material of the present invention described below, a conductive additive, and a binder, and is formed on both surfaces of a negative electrode current collector 41 made of pure copper foil.
[0231] In addition, the positive electrode body 3 has a positive electrode 30 and a positive electrode current collector 31 . The positive electrode 30 is composed of a positive electrode active material, a conductive add...
Embodiment 6
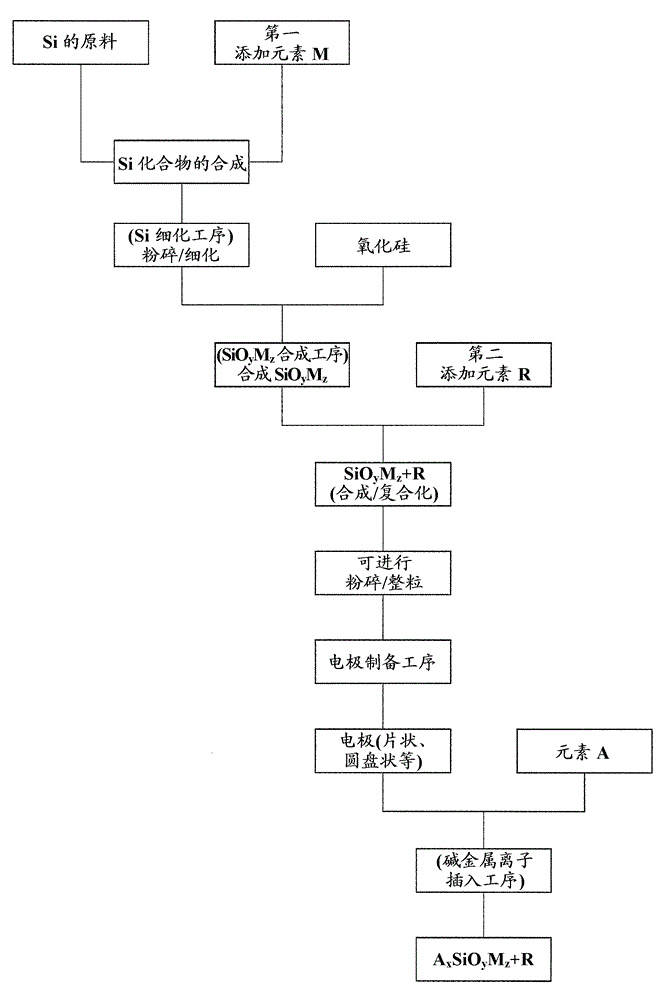
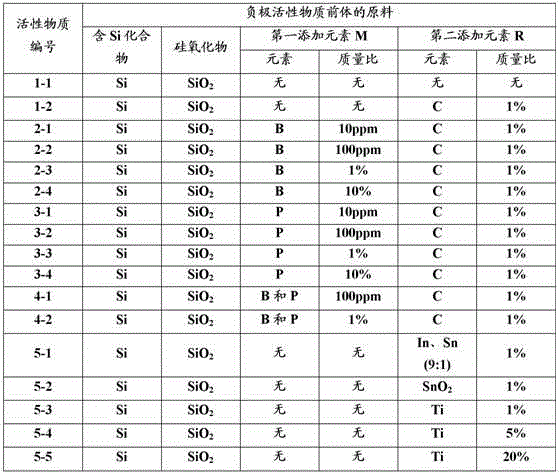
[0270] In addition, in Example 6 (including Example 1) shown in Table 5, C and Ti were added to SiO as a precursor, and the treatment was performed by performing a stabilization process of granulation / sizing, and in addition A non-aqueous electrolyte secondary battery was produced in the same manner as in Example 1 above, and cycle life characteristics were evaluated by the same method.
[0271] In addition, in Examples 7 to 21 shown in Tables 6 and 7, the preparation conditions of the negative electrode active material were changed according to the conditions of each active material number shown in Table 1, and then C, C, and C were added to the SiO as the precursor. Except that Ti was treated through a granulation / sizing stabilization step, a non-aqueous electrolyte secondary battery was prepared in the same manner as in Example 1 above, and cycle life characteristics were evaluated by the same method.
[0272] [Evaluation results]
[0273] Active material numbering 1-1~5-5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com