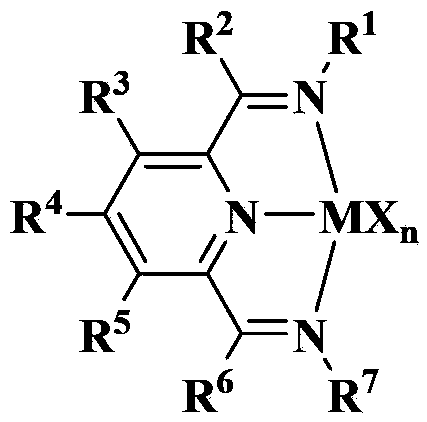
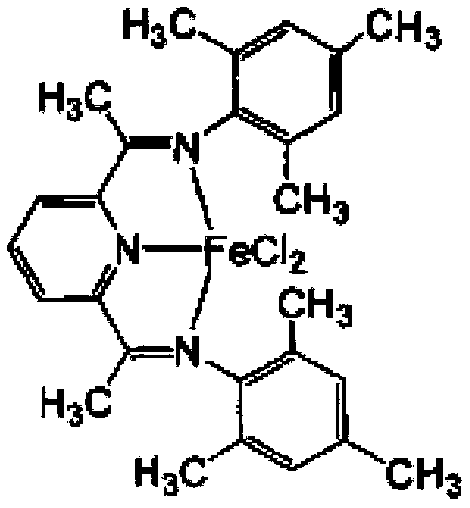
Supported late-transition-metal catalyst used for ethylene polymerization
A late-transition metal and ethylene polymerization technology, which is applied in the field of supported late-transition metal catalysts, can solve the problems of increasing catalyst preparation costs, limiting the industrial application of late-transition metal catalysts, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] (1) Preparation of chlorinated alkyl silicon / silica gel support
[0066] Under the protection of nitrogen, take 10.0 grams of dry silica gel carrier and put it into a glass reactor, add 100 ml of dried hexane, disperse into a suspension, add 1 ml of SiCl 2 (n-Bu) 2 , start stirring, heat up to 30° C., react for 4 hours, and dry in vacuum to obtain a solid powder with good fluidity.
[0067] (2) Preparation of organoaluminum / alkyl silicon chloride / silica gel carrier
[0068] Under nitrogen protection, take 5.0 grams of the modified silica gel carrier obtained above and add it to a glass reactor, add 60 milliliters of dried toluene, disperse into a suspension, and add 18 milliliters of 10 wt% MAO (methylaluminoxane) toluene solution , heated to 50 ° C, stirred for 4 hours, then washed three times with 50 ml × 3 toluene, then washed with hexane, and dried in vacuum to obtain a solid powder with good fluidity, that is, a silica gel carrier containing methylaluminoxane.
...
Embodiment 2
[0078] (1) Preparation of chlorinated hydrocarbyl silicon / silica gel support
[0079] With embodiment 1 step (1), only SiCl in embodiment 1 2 (n-Bu) 2 Change to SiCl 4 .
[0080] (2) Preparation of organoaluminum / chlorinated hydrocarbyl silicon / silica gel carrier
[0081] Same as step (2) of Example 1.
[0082] (3) Preparation of supported transition metal catalyst B
[0083]Same as step (3) of Example 1, only 0.096 grams of (2,6-bis[1-(2,4,6-trimethylbenimine) ethyl]pyridine ferric dichloride in Example 1 is replaced by 0.111 g of [2,6-bis[1-(2,6-diisopropylphenylimine) ethyl]pyridine ferric dichloride] (see below for its structure, see Patent WO9827124A1, Example 8 for its synthesis), The supported transition metal catalyst B was obtained. Characterized by ICP, in the catalyst B, the weight content of Fe was 0.20%, and the weight content of Al was 11.28%.
[0084]
Embodiment 3
[0086] (1) Preparation of chlorinated hydrocarbyl silicon / silica gel support
[0087] Same as (1) preparation method in Example 1.
[0088] (2) Preparation of organoaluminum / chlorinated hydrocarbyl silicon / silica gel carrier
[0089] Same as step (2) of Example 1, only 18 ml of 10% MAO was replaced with 13 ml of 2M diethylaluminum chloride.
[0090] (3) Preparation of supported transition metal catalyst C
[0091] Same as step (3) of Example 1, only 0.096 g of [2,6-bis[1-(2,4,6-trimethylbenimine) ethyl]pyridine ferric dichloride] in Example 1 was replaced It is 0.098 grams [(2,6-bis[1-(2-methyl 6-chloro-phenylimine) ethyl] pyridine ferric dichloride] (see below for its structure, see patent WO9827124A1 for its synthesis, Example 2 ), to obtain the supported transition metal catalyst C. Characterized by ICP, the weight content of Fe in catalyst C was 0.19%, and the weight content of Al was 12.04%.
[0092]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com