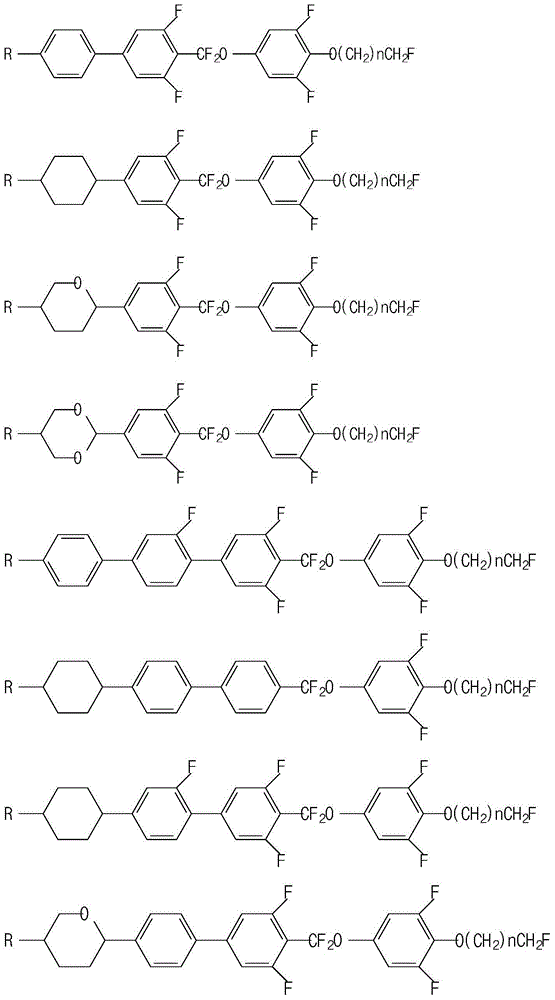
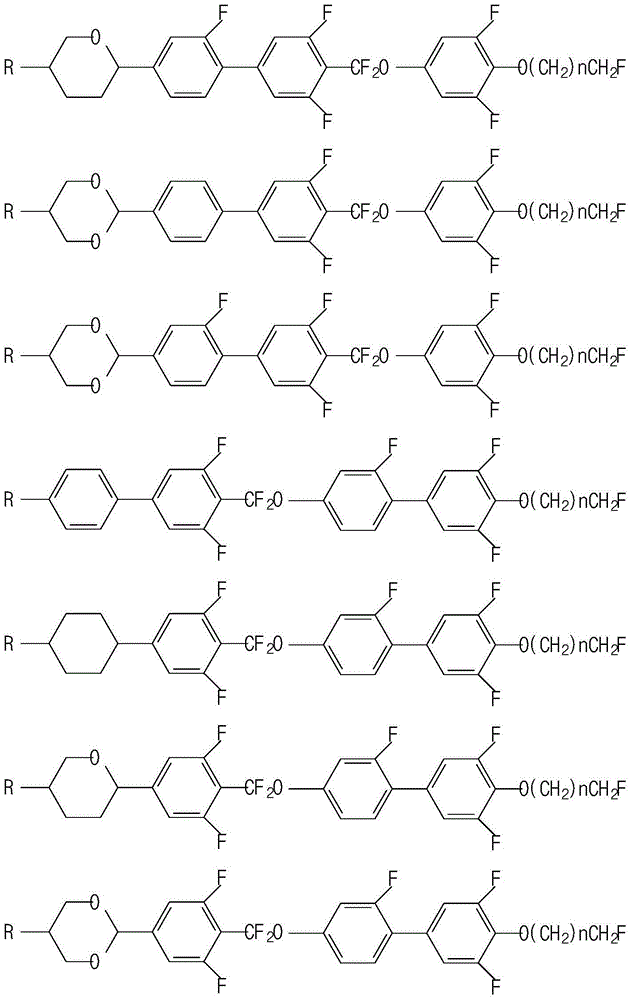
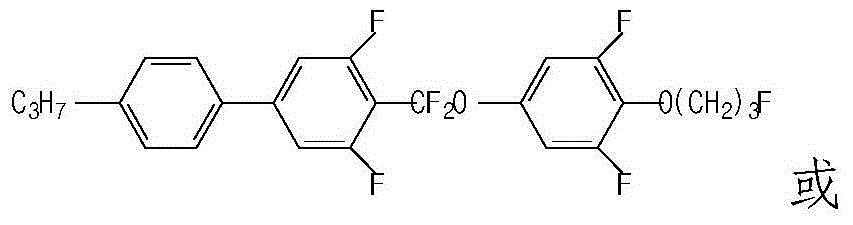
Liquid crystal composition containing difluoromethoxy bridged bond and application thereof
A technology of difluoromethoxy bridges and liquid crystal compounds, which is applied in the field of liquid crystal display materials and can solve problems such as unobtained compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]
[0061] 4-{[3,5-difluoro-4-(3-fluoropropoxy)-phenoxy]-difluoromethyl}-3,5-difluoro-4'-propylbiphenyl (compound 6 )Synthesis
[0062] 1) Synthesis of 5-benzyloxy-1,3-difluoro-2-(3-fluoropropoxy)-benzene (compound 2)
[0063]
[0064] Add 22mL of N,N-dimethylformamide into a 50mL reaction bottle, start stirring, add 5.9g of 2,3-difluoro-4-bromophenol (compound 1), after the solid is completely dissolved, add 10mL of water, 0.25g Tetrabutylammonium bromide, 4.0g anhydrous potassium carbonate. Heat to raise the temperature, add 4.0g of 3-fluorobromopropane dropwise under temperature control at 65-72°C, stir for 4 hours, add 10mL of toluene and 15mL of water, stir for 10 minutes, let stand to separate the liquids, and extract the water phase twice with 5mL×2 toluene ( Stir for 2 minutes, let stand for 5 minutes), and discard the water. All organic phases were combined and washed three times with 10 mL×3 water. The solvent was spin-dried to obtain a white solid, th...
Embodiment 2
[0083] 2-(4-{[3,5-difluoro-4-(3-fluoropropoxy)-phenoxy]-difluoromethyl}-3,5-difluorophenyl)-5-propyl Synthesis of -[1,3]dioxane (Compound 9)
[0084] 1) Synthesis of onium trifluoromethanesulfonate (compound 8)
[0085]
[0086] Add 106g of 2,6-dioxo-4-(5-propyl-[1,3]dioxocyclohexane-2-yl)-benzoic acid (compound 7), 47mL of 1,3-propanedi Mercaptan, 42mL of trifluoromethanesulfonic acid, 145mL of toluene and 145mL of isooctane, install a water separator at one port, raise the temperature to reflux, react for 6 hours, slowly cool to 0°C, and suction filter to obtain a solid. After drying, proceed to the next step of feeding.
[0087] 2) 4-{[3,5-difluoro-4-(3-fluoropropoxy)-phenoxy]-difluoromethyl}-3,5-difluoro-4'-propyl biphenyl ( Compound 9) Synthesis
[0088]
[0089] Add 200mL of dichloromethane, 39mL of triethylamine and 57.7g of 3,5-difluoro-4-(3-fluoropropoxy)-phenol (compound 3) into a 2L three-necked flask, cool down to 20°C, add 120g of trifluoro A solution c...
Embodiment 3
[0098] 4-{[3,5-Difluoro-4-(3-fluoropropoxy)-phenoxy]-difluoromethyl}-3,5,2'-trifluoro-4''-propyl- Synthesis of [1,1'; 4',1'']terphenyl (compound 12)
[0099] 1) Synthesis of onium trifluoromethanesulfonate (compound 11)
[0100]
[0101]
[0102] Add 137g 3,5,2'-trifluoro-4''-propyl-[1,1'; 4',1'']terphenyl-4-carboxylic acid (compound 10), 47mL1,3- Propanedithiol, 42mL trifluoromethanesulfonic acid, 145mL toluene, and 145mL isooctane were installed with a water separator at one port, heated to reflux, reacted for 6 hours, cooled slowly to 0°C, and filtered with suction to obtain a solid. After drying, proceed to the next step of feeding.
[0103] 2) 4-{[3,5-difluoro-4-(3-fluoropropoxy)-phenoxy]-difluoromethyl}-3,5-difluoro-4'-propyl biphenyl ( Compound 12) Synthesis
[0104]
[0105] Add 200mL of dichloromethane, 39mL of triethylamine and 57.7g of 3,5-difluoro-4-(3-fluoropropoxy)-phenol (compound 3) into a 2L three-necked flask, cool down to 20°C, and add 142g of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com