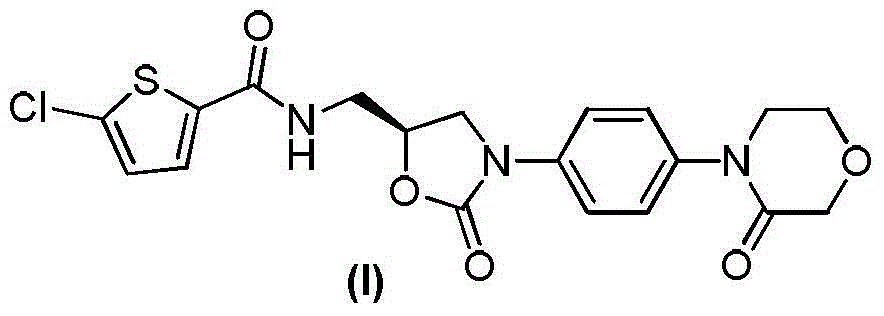
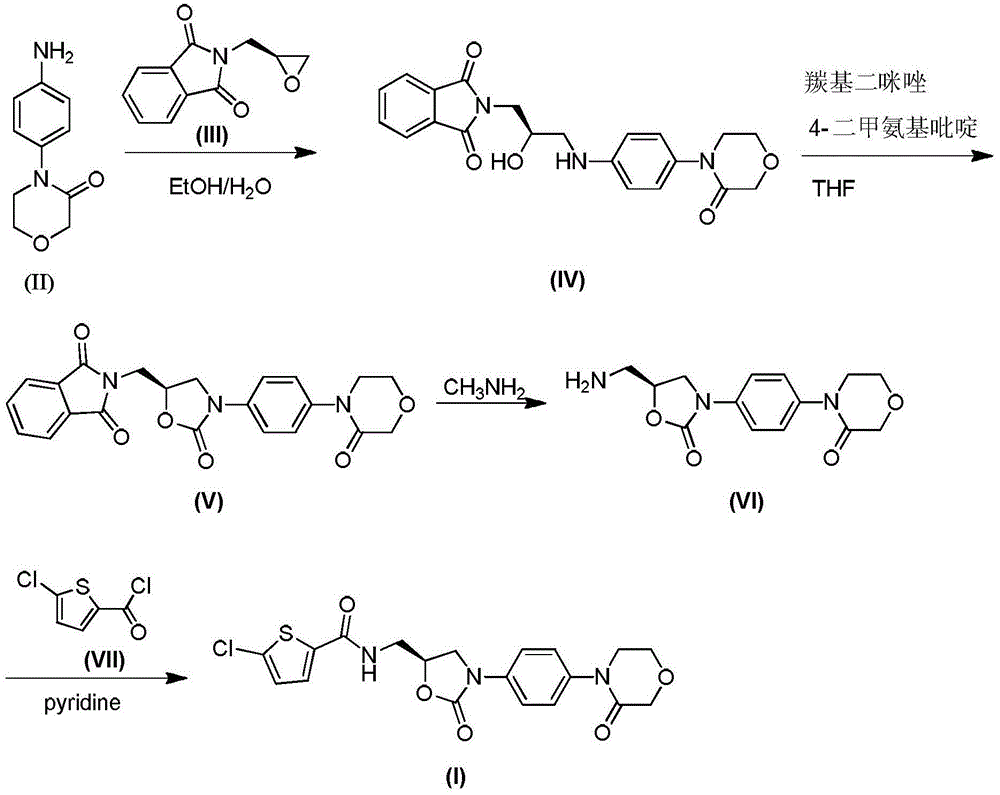
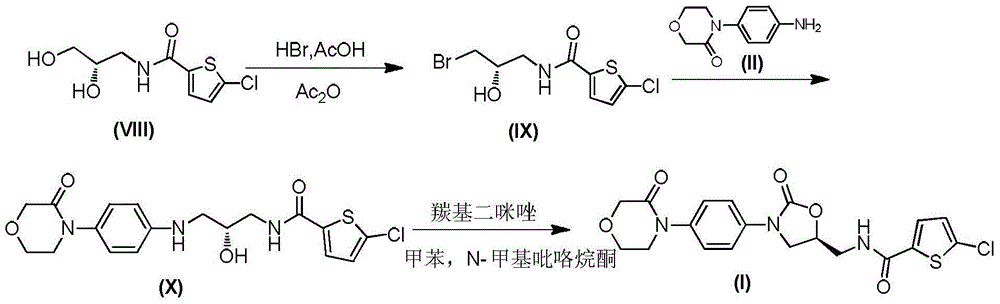
Preparation method of rivaroxaban
A technology of rivaroxaban and organic solvents, applied in the field of medicine, can solve problems such as inability to carry out industrial production, incomplete substrate reaction, toxicity yield, etc., and achieve the effect of low cost, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] a) Preparation of 2-((2R)-2-hydroxy-3-{[4-(3-oxo-4-morpholinyl)phenyl]amino}propyl)-1H-isoindole-1,3 (2H)-Diketone (IV)
[0033] Add absolute ethanol (1.48kg) and process water (467g) in the reaction bottle of 3L, start stirrer and stir, then add 4-(4-aminophenyl)-3-morpholinone (II) (62.3 g) and 2-[(2S)-2-oxiranylmethyl]-1H-isoindole-1,3(2H)-2-ketone (III) (65.9g), start heating to 70°C, React for 4 hours, stop heating, cool down to 35°C, filter with suction, rinse the solid with 90% ethanol (150g), pump the mother liquor back into the reaction flask, evaporate 1kg of solvent, and add 2-[(2S)- 2-Oxiranylmethyl]-1H-isoindole-1,3(2H)-2-ketone (III) (32.9g), heated to 70°C, reacted for 4 hours, stopped heating, cooled to 30°C , suction filtration, the solid was rinsed with 90% ethanol (100 g), and the filtrate was treated as waste liquid, and the solids obtained by suction filtration twice were combined and dried. 121.8 g of product were obtained, purity: 96%, melting ...
Embodiment 2
[0042] a) Preparation of 2-((2R)-2-hydroxy-3-{[4-(3-oxo-4-morpholinyl)phenyl]amino}propyl)-1H-isoindole-1,3 (2H)-Diketone (IV)
[0043] Add absolute ethanol (1.48kg) and process water (467g) in the reaction bottle of 3L, start stirrer and stir, then add 4-(4-aminophenyl)-3-morpholinone (II) (62.3 g) and 2-[(2S)-2-oxiranylmethyl]-1H-isoindole-1,3(2H)-2-ketone (III) (65.9g), start heating to 70°C, React for 4 hours, stop heating, cool down to 25°C, filter with suction, rinse the solid with 90% ethanol (150g), pump the mother liquor back into the reaction flask, distill 1kg of solvent, add 2-[(2S)- 2-Oxiranylmethyl]-1H-isoindole-1,3(2H)-2-ketone (III) (32.9g), heated to 70°C, reacted for 4 hours, stopped heating, cooled to 35°C , suction filtration, the solid was rinsed with 90% ethanol (100 g), and the filtrate was treated as waste liquid, and the solids obtained by suction filtration twice were combined and dried. 117.8 g of product were obtained, purity: 90%, melting point:...
Embodiment 3
[0052] a) Preparation of 2-((2R)-2-hydroxy-3-{[4-(3-oxo-4-morpholinyl)phenyl]amino}propyl)-1H-isoindole-1,3 (2H)-Diketone (IV)
[0053] Add absolute ethanol (1.48kg) and process water (467g) in the reaction flask of 3L, start to stir, then add 4-(4-aminophenyl)-3-morpholinone (II) (62.3g) and 2-[(2S)-2-oxiranylmethyl]-1H-isoindole-1,3(2H)-2-ketone (III) (65.9g), start heating to 70°C, react for 4 hours , stop heating, cool down to 35°C, filter with suction, rinse the solid with 90% ethanol (150g), pump the mother liquor back into the reaction flask, evaporate 1kg of solvent, and add 2-[(2S)-2-cyclo Oxyethylmethyl]-1H-isoindole-1,3(2H)-2-ketone (III) (39.54g), heated to 70°C, reacted for 4 hours, stopped heating, cooled to 30°C, and suction filtered , The solid was rinsed with 90% ethanol (100 g), the filtrate was treated as waste liquid, and the solid obtained by suction filtration twice was combined and dried. 122.9 g of product were obtained, purity: 96%, melting point: 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com