Benzene-ring-containing modified acrylamide water-soluble amphoteric polymer and preparation method thereof
A technology of amphoteric polymer and acrylamide, which is applied in the field of water-soluble polymer synthesis to achieve the effect of improving temperature resistance, wide application potential and reducing surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
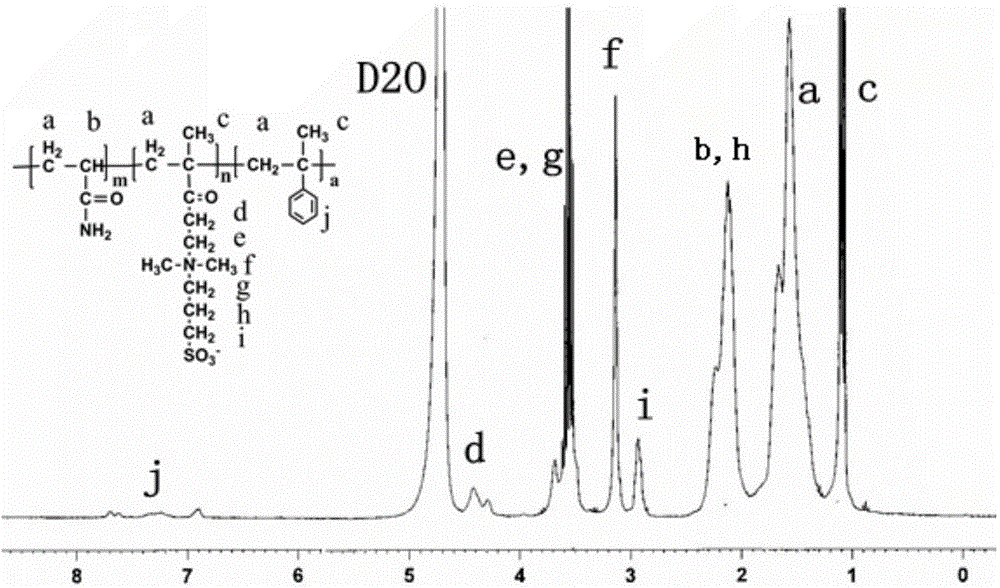
[0049] The preparation of the acrylamide water-soluble amphoteric polymer containing benzene ring modification, the steps are as follows:
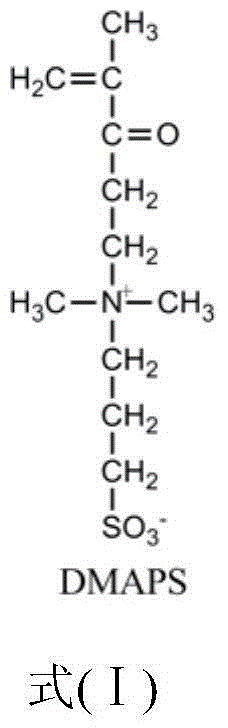
[0050] (1) Weigh 3.36g (0.04735mol) of acrylamide and 0.6975g (0.0025mol) of methacryloyloxyethyl-N,N-dimethylpropanesulfonate amphoteric monomer and dissolve them in water. Nitrogen tube, condenser tube and a 100mL three-neck flask with a stirring magnet, stir at room temperature until completely dissolved;
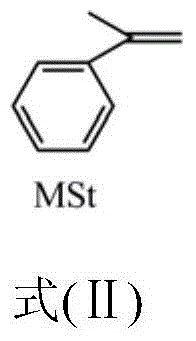
[0051] (2) Add 0.0177g (0.15mmol) hydrophobic monomer methylstyrene and 1.44g (0.1mol.L) to the system obtained in step (1) -1 ) emulsifier sodium lauryl sulfate, feed nitrogen and fully stir at room temperature;
[0052] (3) Add 0.054g (4mmol.L) to the system obtained in step (2) -1 ) Initiator potassium persulfate, after being stirred evenly, placed in a constant temperature water bath at 25° C., and deoxygenated with nitrogen gas for 0.5 hour;
[0053](4) The system obtained in step (3) was reacted at 65°C for 6 hours to obtain...
Embodiment 2
[0060] The preparation of the acrylamide water-soluble amphoteric polymer containing benzene ring modification, the steps are as follows:
[0061] As described in Example 1, the difference is that the dosage of acrylamide in step (1) is 3.3548g (0.04725mol), and the dosage of hydrophobic monomer methylstyrene in step (2) is 0.0295g (0.25mmol).
[0062] 3.632 g of water-soluble polymer powder containing benzene ring modified acrylamide was obtained, with a yield of ≥89%.
Embodiment 3
[0064] The preparation of the acrylamide water-soluble amphoteric polymer containing benzene ring modification, the steps are as follows:
[0065] (1) Weigh 3.34765g (0.04715mol) of acrylamide and 0.6975g (0.0025mol) of methacryloyloxyethyl-N,N-dimethylpropanesulfonate amphoteric monomer and dissolve it in water. Nitrogen tube, condenser tube and a 100mL three-neck flask with a stirring magnet, stir at room temperature until completely dissolved;
[0066] (2) Add 0.04136g (0.35mmol) hydrophobic monomer methylstyrene and 1.44g (0.1mol.L) to the system obtained in step (1) -1 ) emulsifier sodium lauryl sulfate, feed nitrogen and fully stir at room temperature;
[0067] (3) Add 0.054g (4mmol.L) to the system obtained in step (2) -1 ) Initiator potassium persulfate, after being stirred evenly, placed in a constant temperature water bath at 25° C., and deoxygenated with nitrogen gas for 0.5 hour;
[0068] (4) The system obtained in step (3) was reacted at 70°C for 6 hours to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal degradation temperature | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com