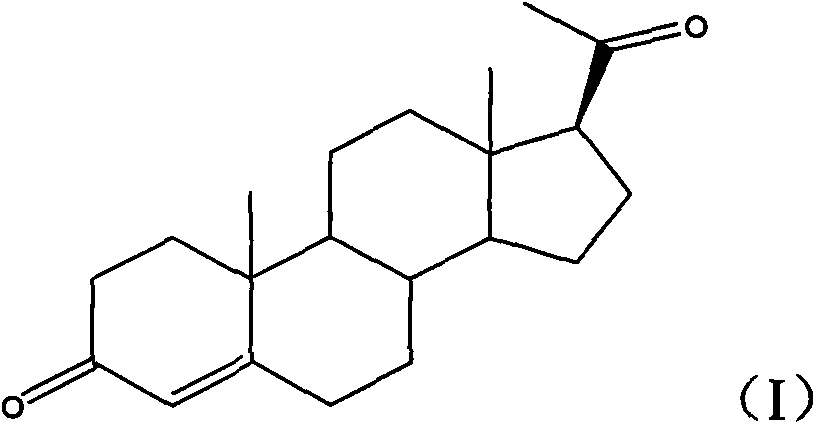
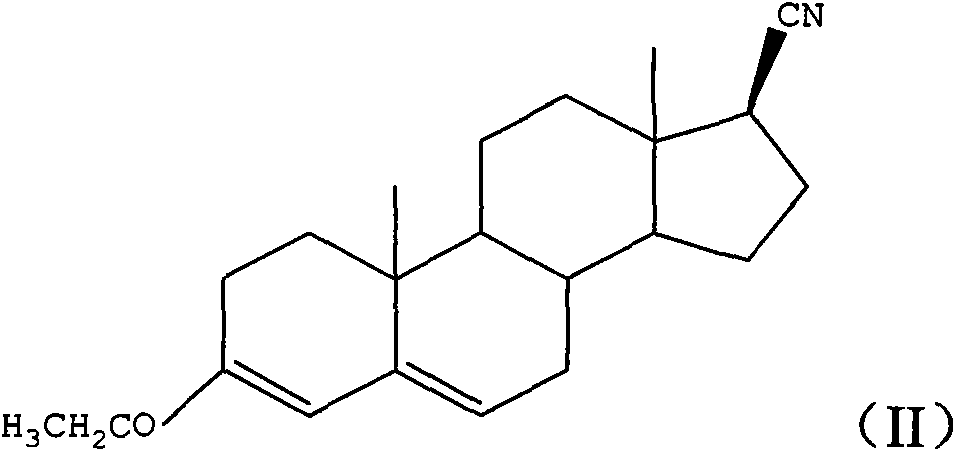
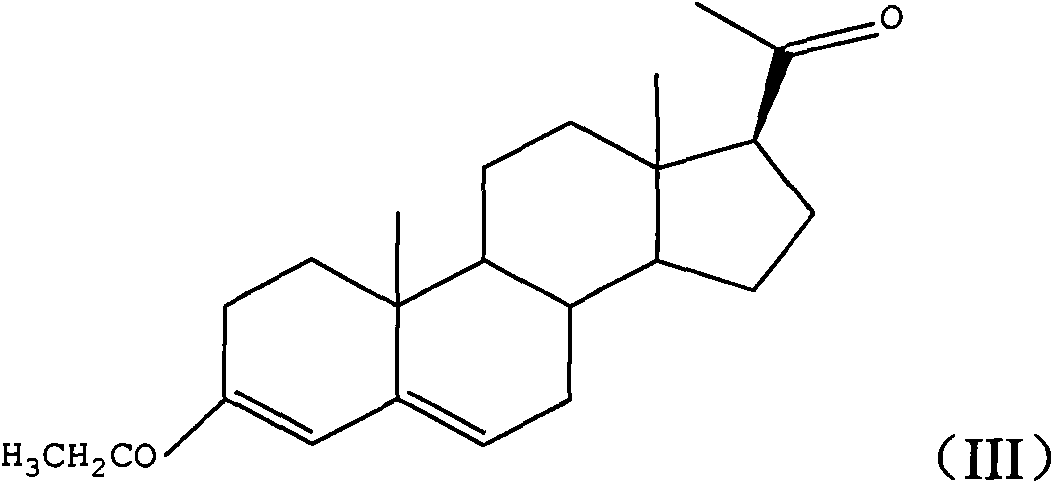
Progesterone preparation method
A progesterone and product technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of unavailable raw materials, low yield, high progesterone price, etc., and achieve low price, high yield and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The embodiment of the present invention discloses a preparation method of progesterone, the method comprises the following steps:
[0028] a), under strong alkali conditions, 3-ethoxy-androst-3,5-dien-17-one and p-toluenesulfonylmethyl isonitrile react in an organic solvent to obtain formula (II) structure first intermediate product;
[0029]
[0030] b), the first intermediate product reacts with the Grignard reagent to obtain the second intermediate product of the formula (III) structure;
[0031]
[0032] c), the second intermediate product is hydrolyzed to obtain progesterone.
[0033] The preparation method of progesterone provided by the embodiment of the present invention is based on 3-ethoxyandrost-3,5-dien-17-one, which is relatively low in cost and easy to obtain in the market, as a raw material. First, the 17-keto group of the raw material One-step conversion to 17β-cyano group (step a above), then conversion of 17β-cyano group to 17β-acetyl group (st...
Embodiment 1
[0054] Example 1 Preparation of the first intermediate
[0055] Under the protection of nitrogen flow, 25g 3-ethoxy androst-3,5-dien-17-one and 45g potassium tert-butoxide were added to 1L ethylene glycol dimethyl ether and 300ml tert-butanol, and cooled to -5°C, add dropwise a solution made of 23.43g p-toluenesulfonylmethyl isonitrile and 140ml ethylene glycol dimethyl ether, after the dropwise addition is completed, return the temperature to room temperature, stir for 3h, and pour half-saturated chlorine In the sodium chloride aqueous solution, precipitates were precipitated, filtered, washed with water, and dried under reduced pressure to obtain 23.8 g of white crystals with a yield of 92%.
[0056] The proton nuclear magnetic resonance spectrum of product is as follows:
[0057] 1 H-NMR(CDCl3) 0.95(s, 3H, 18-CH 3 )
[0058] 1.20(s, 3H, 19-CH 3 )
[0059] 5.71 (s, 1H, 4-H)
[0060] It can be seen that the first intermediate product prepa...
Embodiment 2
[0062] Embodiment two prepares the first intermediate
[0063] Under the protection of nitrogen flow, 25g 3-ethoxy androst-3,5-dien-17-one and 45g potassium tert-butoxide were added to 1L ethylene glycol dimethyl ether and 300ml tert-butanol, and cooled to -5°C, add dropwise a solution composed of 22g p-toluenesulfonylmethyl isonitrile and 140ml ethylene glycol dimethyl ether, after the dropwise addition is complete, return the temperature to room temperature, stir for 3h, pour into half-saturated chlorinated In the aqueous sodium solution, precipitates were precipitated, filtered, washed with water, and dried under reduced pressure to obtain 23.3 g of white crystals with a yield of 90%. The proton nuclear magnetic resonance spectrum of product is as follows:
[0064] 1 H-NMR(CDCl3) 0.95(s, 3H, 18-CH 3 )
[0065] 1.20(s, 3H, 19-CH 3 )
[0066] 5.71 (s, 1H, 4-H)
[0067] It can be known that the first intermediate product prepared in this ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com