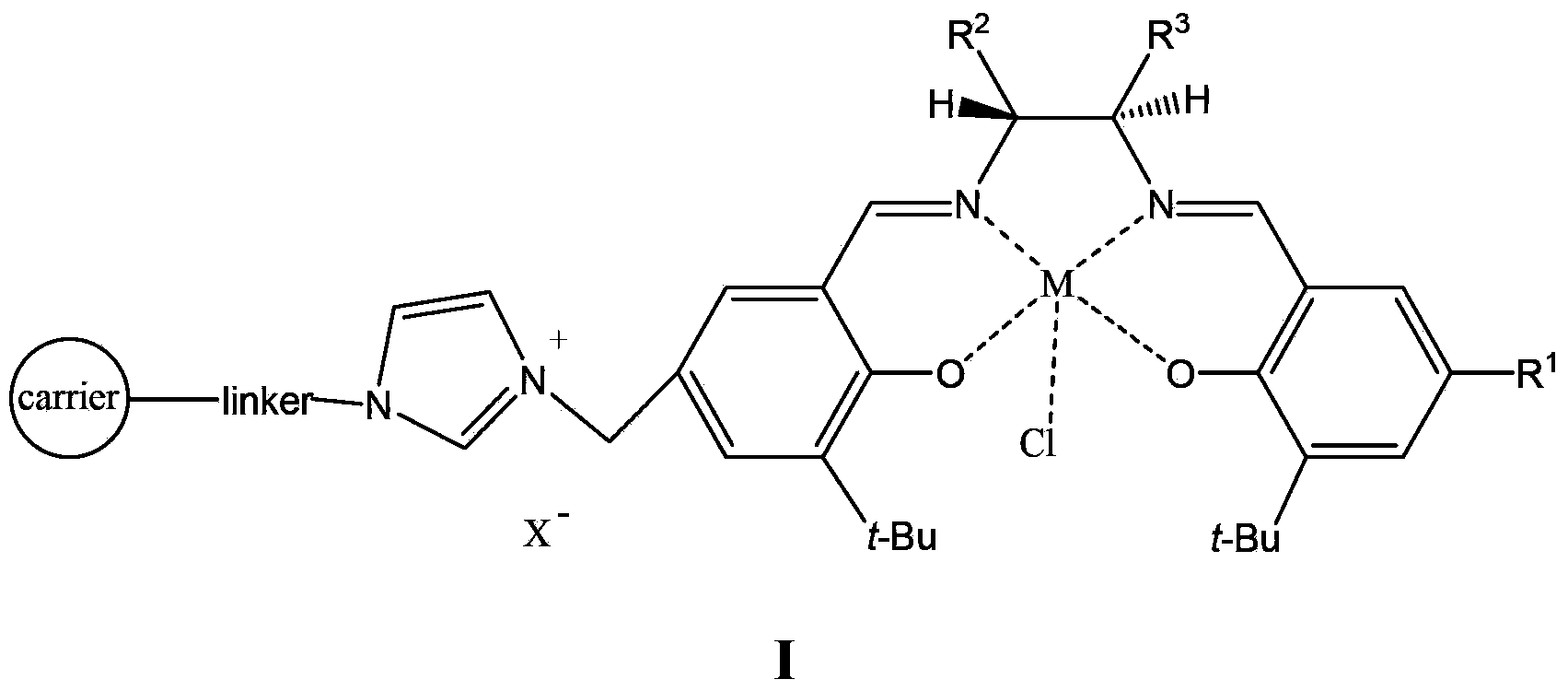
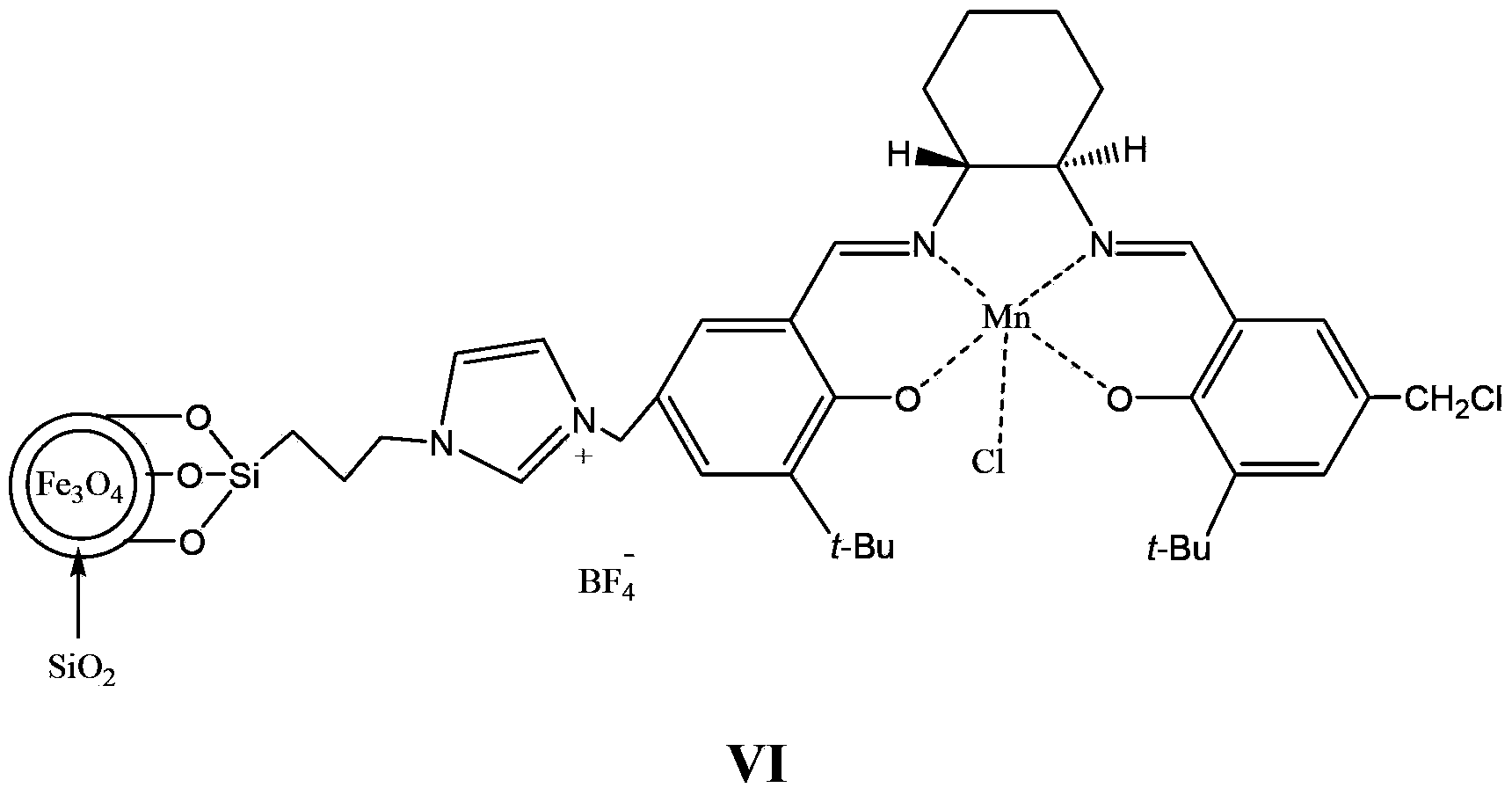
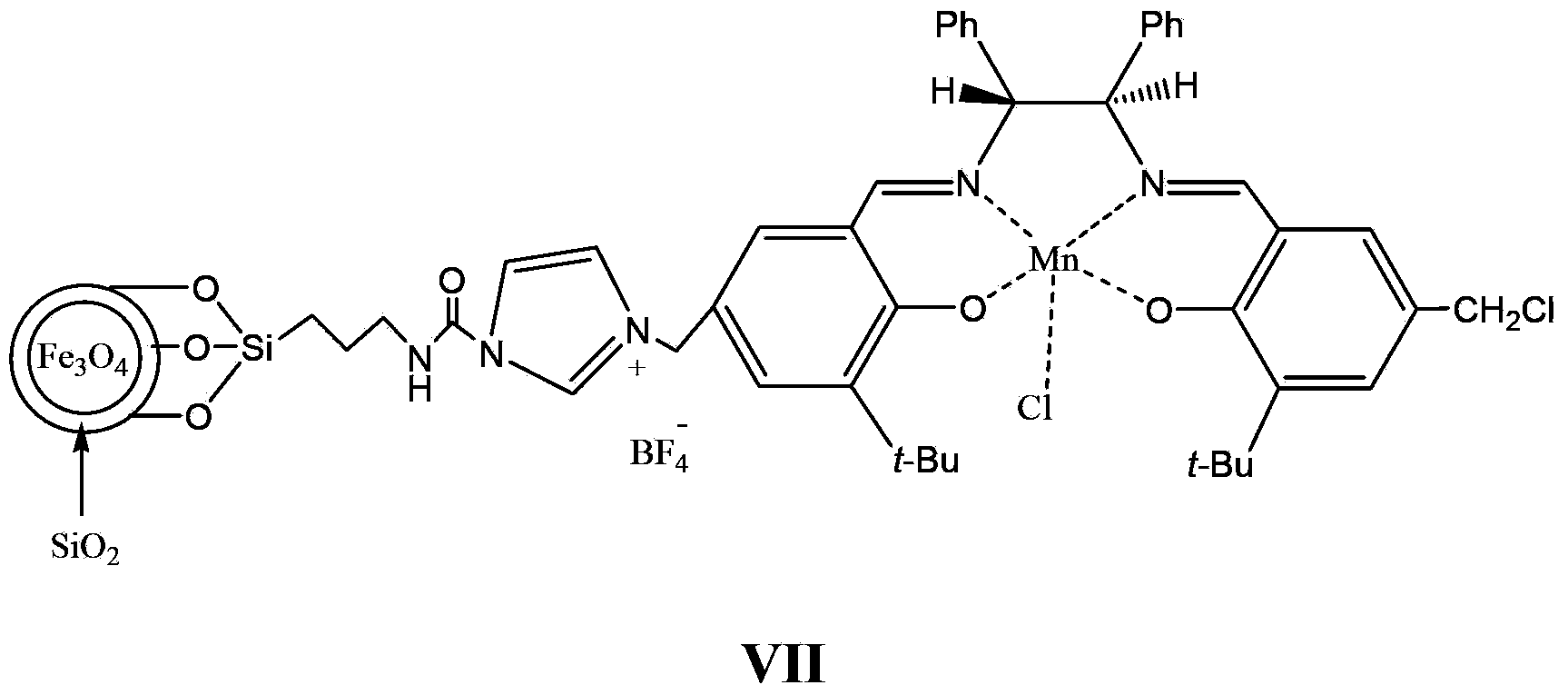
Supported catalyst and preparation method thereof
A technology of supported catalysts and compounds, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc. Rate and enantioselectivity decline and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080]
[0081] The experimental device is mainly a three-neck flask. In order to stir fully, mechanical stirring is used, and the system is protected with nitrogen. Place the accurately weighed compound of formula 5 (14.8g, 10mmol, 0.68mmol / g as imidazole), the compound of formula 1 (12mmol) and 80mL of toluene in a flask, place the flask in an oil bath, stir at a high speed, and reflux the reaction After 24 hours, add an external magnetic field, pour the liquid, and then add 15 mmol of Mn(OAc) 2 4H 2 O, 60mL absolute ethanol, after reflux for 1 hour, add lithium chloride (15mmol) to continue the reflux reaction for 1 hour, add a magnetic field, pour the liquid, add 100mL methanol, NaBF 4 (15 mmol) after reflux reaction for 10 hours, a magnetic field was added, the liquid was poured, and the obtained solid liquid was washed 3 times with anhydrous ether, and dried in vacuum at 60° C. for 5 hours to obtain 14.1 g of a light yellow solid. After elemental analysis, the loadi...
Embodiment 2
[0083]
[0084] The experimental device is mainly a three-neck flask. In order to stir fully, mechanical stirring is used, and the system is protected with nitrogen. Place the accurately weighed compound of formula 5 (14.8g, 10mmol, 0.68mmol / g in terms of imidazole), the compound of formula 2 (15mmol) and 80mL of toluene in a flask, place the flask in an oil bath, stir at a high speed, and reflux the reaction After 24 hours, add an external magnetic field, pour the liquid, and then add 15 mmol of Mn(OAc) 2 4H 2 O, 80mL of absolute ethanol, add lithium chloride (15mmol) after reflux for 1 hour and continue to reflux for 1 hour, add a magnetic field, pour the liquid, add 100mL of methanol, LiNTf 2 (15 mmol) after reflux reaction for 24 hours, a magnetic field was added, the liquid was poured, and the obtained solid liquid was washed three times with anhydrous ether, and dried in vacuum at 60° C. for 5 hours to obtain 14.6 g of a light yellow solid. After elemental analysis,...
Embodiment 3
[0086]
[0087] The experimental device is mainly a three-neck flask. In order to stir fully, mechanical stirring is used, and the system is protected with nitrogen. Place accurately weighed compound of formula 6 (15.6g, 10mmol, 0.64mmol / g in terms of imidazole), compound of formula 3 (13mmol) and 80mL of toluene in a flask, place the flask in an oil bath, stir at a high speed, and reflux the reaction After 24 hours, add an external magnetic field, pour the liquid, and then add 15 mmol of Mn(OAc) 2 4H 2 O, 80mL absolute ethanol, after reflux for 1 hour, add lithium chloride (15mmol) to continue the reflux reaction for 1 hour, add a magnetic field, pour the liquid, add 110mL methanol, KPF 6(15 mmol) after reflux reaction for 12 hours, a magnetic field was added, the liquid was poured, and the obtained solid liquid was washed 3 times with anhydrous ether, and dried in vacuum at 60° C. for 5 hours to obtain 13.9 g of a light yellow solid. According to elemental analysis, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com