Preparation and application of trifluoroacetyl anabasine compounds with insecticidal activity
A technology of compounds and compositions, applied in the fields of pesticides, organic chemistry, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
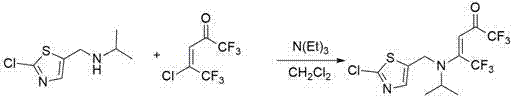
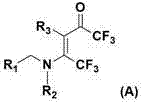
[0045] The structural formula of some compounds of the present invention is one of the specific compounds listed in Table 1:
[0046] Table 1: Representative compounds represented by structural formula (A)
[0047]
[0048] Continuation
[0049]
[0050] Continuation
[0051]
Embodiment 2
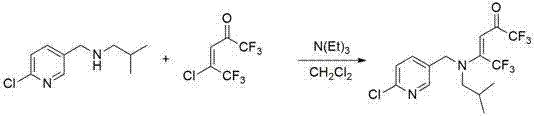
[0052] Example 2. Preparation of the compound numbered 1 in embodiment 1
[0053]
[0054] Under ice-bath conditions, add 0.31 g (2.2 mmol) of (6-chloropyridin-3-yl)-methylamine, 0.45 g (2 mmol) of 4-chloro-1,1, 1,5,5,5-Hexafluoro-3-en-2-one, 0.3 mL (2.2 mmol) of triethylamine and 15 mL of dichloromethane, stirred reaction. The reaction was tracked by thin layer chromatography (TLC). When the reactant 4-chloro-1,1,1,5,5,5-hexafluoro-3-en-2-one disappeared, the reaction was stopped, and the crude product of the solvent was removed by rotary evaporation. Using dichloromethane and methanol as eluents for column chromatography separation, a light yellow powdery solid was obtained with a yield of 65%;
[0055] 1 H NMR (400 Mz, CDCl 3 ): δ 8.28 (d, J = 2.2 Hz, 1H, Py- H ), 7.51 (dd, J = 8.2 Hz, J = 2.4 Hz, 1H, Py- H ), 7.39 (d, J = 8.4 Hz, 1H, Py- H ), 6.01 (s, 1H), 4.62 (s, 2H, Py-C H 2 -), 4.10 (s, 1H).
Embodiment 3
[0056] Example 3. Preparation of the compound numbered 2 in embodiment 1
[0057]
[0058] Under ice-bath conditions, add 0.35 g (2.2 mmol) of N-((6-chloropyridin-3-yl)-methyl)methanamine, 0.45 g (2 mmol) of 4- Chloro-1,1,1,5,5,5-hexafluoro-3-en-2-one, 0.3 mL (2.2 mmol) of triethylamine and 15 mL of dichloromethane, stirred reaction. The reaction was tracked by thin layer chromatography (TLC). When the reactant 4-chloro-1,1,1,5,5,5-hexafluoro-3-en-2-one disappeared, the reaction was stopped, and the crude product of the solvent was removed by rotary evaporation. Using dichloromethane and methanol as eluents for column chromatography separation, a light yellow powdery solid was obtained with a yield of 82%;
[0059] 1 H NMR (400 Mz, CDCl 3 ): δ 8.27 (d, J = 2.0 Hz, 1H, Py- H ), 7.50 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H, Py- H ), 7.37 (d, J = 8.4 Hz, 1H, Py- H ), 6.00 (s, 1H), 4.60 (s, 2H, Py-C H 2 -), 2.98 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com