Preparation method of canagliflozin
An intermediate and molar ratio technology, applied in the field of medicine, can solve the problems of unfavorable industrialization reaction, unfavorable refining and impurity removal, poor product quality, etc., and achieve the effect of simple post-treatment process, reduced complexity, and simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
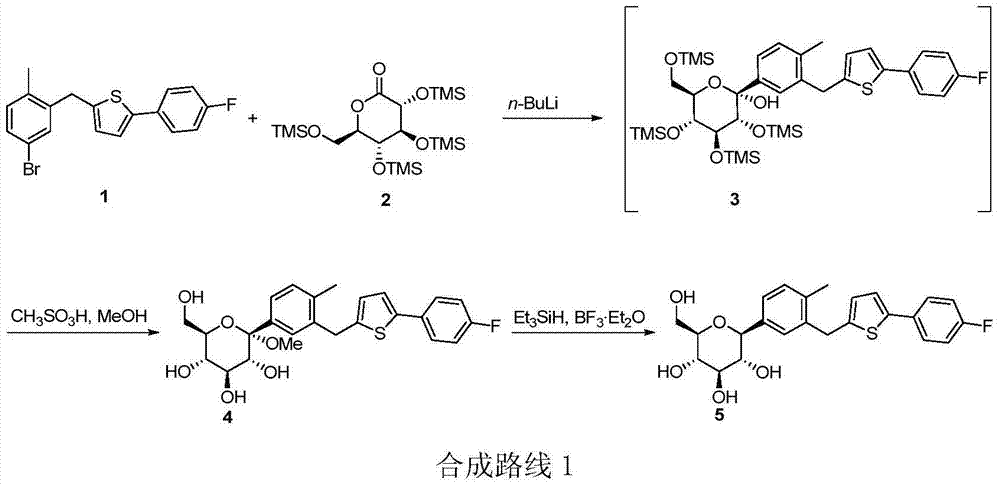
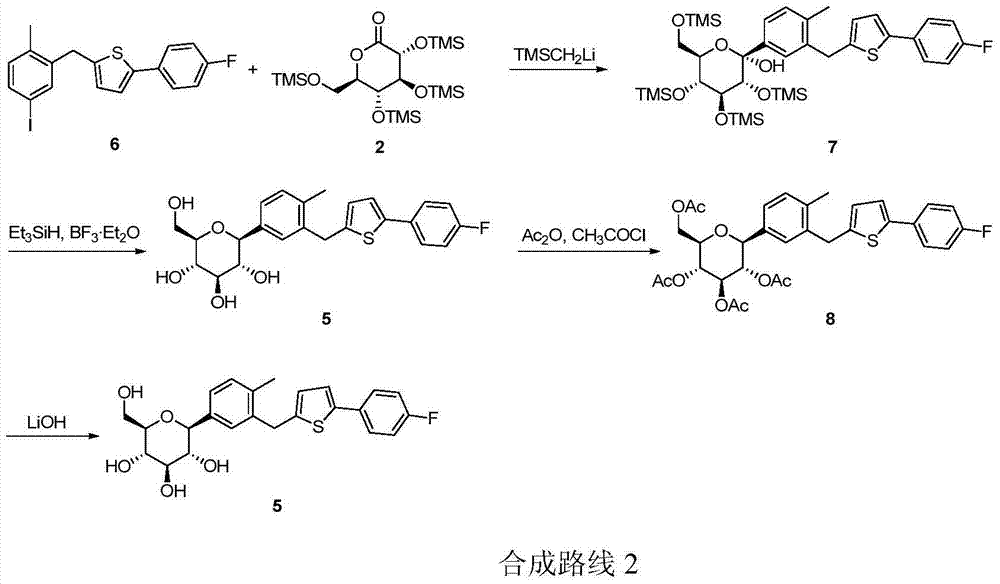
Image
Examples
Embodiment 1
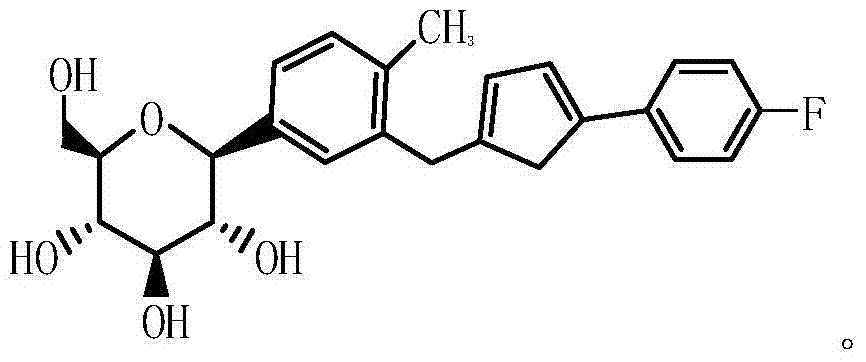
[0048] Add 20g of the main raw material SM1 methyl 1-C-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methylphenyl to a reaction flask containing 150ml of DMF )-D-glucopyranoside, stirred until the feed liquid was clarified, slowly added 29.6g (5 times the amount) of benzoyl chloride after the feed liquid was clarified, and reacted at room temperature for 3h; TLC monitored the completion of the reaction and added 100ml of dichloromethane and 100ml of water , stirred and extracted, and the upper aqueous phase was stirred and extracted twice with 80ml and 60ml of dichloromethane respectively, and the organic phase was combined and washed once with 100ml saturated saline water, and the organic phase was dried with anhydrous sodium sulfate for 30min, and then the feed liquid was evaporated to Drying yielded 37.6 g of intermediate I (molecular weight 890).
[0049] After the intermediate I obtained in the previous step was dissolved in 120ml of acetonitrile, 10.5g of trimethyl phosphi...
Embodiment 2
[0052] Add 20g of the main raw material SM1 methyl 1-C-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methylphenyl to a reaction flask containing 150ml of DMF )-D-glucopyranoside, stirred until the feed liquid was clear, and slowly added 29.6g (5 times the amount) of benzoyl chloride after the feed liquid was dissolved, and reacted at room temperature for 3h; TLC monitored the completion of the reaction and added 100ml dichloromethane and 100ml water, stirred and extracted, and the upper aqueous phase was stirred and extracted twice with 80ml and 60ml of dichloromethane respectively, and the organic phase was combined and washed once with 100ml saturated saline water, and the organic phase was dried with anhydrous sodium sulfate for 30min, and then the feed liquid was evaporated To dryness, 37.6 g of intermediate I were obtained.
[0053] After the intermediate I obtained in the previous step was dissolved in 120ml of acetonitrile, 11.6g of trimethyl phosphite (2.2 times the amou...
Embodiment 3
[0056] Add 20g of the main raw material SM1 methyl 1-C-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methylphenyl to a reaction flask containing 150ml of DMF )-D-glucopyranoside, stir until the feed solution is clear, and slowly add 23.7g (4 times the amount) of benzoyl chloride after the feed solution is clarified, and react at room temperature for 3h; TLC monitors the completion of the reaction and then adds 100ml dichloromethane and 100ml water, stirred and extracted, and the upper aqueous phase was stirred and extracted twice with 80ml and 60ml of dichloromethane respectively, and the organic phase was combined and washed once with 100ml saturated saline water, and the organic phase was dried with anhydrous sodium sulfate for 30min, and then the feed liquid was evaporated To dryness, 37.6 g of intermediate I were obtained.
[0057] After the intermediate I obtained in the previous step was dissolved in 120ml of acetonitrile, 11.6g of trimethyl phosphite (2.2 times the amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com